Combination Therapies Targeting ALK-aberrant Neuroblastoma in Preclinical Models
- PMID: 36602782
- PMCID: PMC10068437
- DOI: 10.1158/1078-0432.CCR-22-2274
Combination Therapies Targeting ALK-aberrant Neuroblastoma in Preclinical Models
Abstract
Purpose: ALK-activating mutations are identified in approximately 10% of newly diagnosed neuroblastomas and ALK amplifications in a further 1%-2% of cases. Lorlatinib, a third-generation anaplastic lymphoma kinase (ALK) inhibitor, will soon be given alongside induction chemotherapy for children with ALK-aberrant neuroblastoma. However, resistance to single-agent treatment has been reported and therapies that improve the response duration are urgently required. We studied the preclinical combination of lorlatinib with chemotherapy, or with the MDM2 inhibitor, idasanutlin, as recent data have suggested that ALK inhibitor resistance can be overcome through activation of the p53-MDM2 pathway.
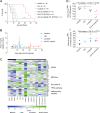
Experimental design: We compared different ALK inhibitors in preclinical models prior to evaluating lorlatinib in combination with chemotherapy or idasanutlin. We developed a triple chemotherapy (CAV: cyclophosphamide, doxorubicin, and vincristine) in vivo dosing schedule and applied this to both neuroblastoma genetically engineered mouse models (GEMM) and patient-derived xenografts (PDX).
Results: Lorlatinib in combination with chemotherapy was synergistic in immunocompetent neuroblastoma GEMM. Significant growth inhibition in response to lorlatinib was only observed in the ALK-amplified PDX model with high ALK expression. In this PDX, lorlatinib combined with idasanutlin resulted in complete tumor regression and significantly delayed tumor regrowth.
Conclusions: In our preclinical neuroblastoma models, high ALK expression was associated with lorlatinib response alone or in combination with either chemotherapy or idasanutlin. The synergy between MDM2 and ALK inhibition warrants further evaluation of this combination as a potential clinical approach for children with neuroblastoma.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


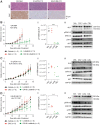

![Figure 6. Combination effects of lorlatinib and idasanutlin in ALK-altered neuroblastoma PDX models. A, GR-NB4 (B) IC-pPDX-112 (C) HSJD-NB-012 PDX models were treated with vehicle control, idasanutlin, lorlatinib, or idasanutlin and lorlatinib combination. I, Tumor volumes were monitored during treatment [with (A.ii) inset of GR-NB4 PDX up to day 10]. (A.iii, B.ii, and C.ii) pY1586/total ALK measured by immunoassay in tumor lysates taken at the end of the experiment (mean of two technical replicates). One-way ANOVA: A.iii: P = <0.0001; B.ii: P = 0.0331; C.ii: P = 0.0020.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/69b8/10068437/fe3a167e606b/1317fig6.gif)
References
-
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. . Neuroblastoma. Nat Rev Dis Primers 2016;2:16078. - PubMed
-
- Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984;224:1121–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

