Insulin-like growth factor 1 reduces coronary atherosclerosis in pigs with familial hypercholesterolemia
- PMID: 36602878
- PMCID: PMC9990768
- DOI: 10.1172/jci.insight.165713
Insulin-like growth factor 1 reduces coronary atherosclerosis in pigs with familial hypercholesterolemia
Abstract
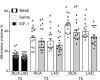
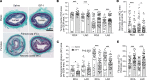
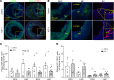
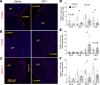
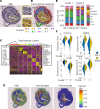
Although murine models of coronary atherosclerotic disease have been used extensively to determine mechanisms, limited new therapeutic options have emerged. Pigs with familial hypercholesterolemia (FH pigs) develop complex coronary atheromas that are almost identical to human lesions. We reported previously that insulin-like growth factor 1 (IGF-1) reduced aortic atherosclerosis and promoted features of stable plaque in a murine model. We administered human recombinant IGF-1 or saline (control) in atherosclerotic FH pigs for 6 months. IGF-1 decreased relative coronary atheroma in vivo (intravascular ultrasound) and reduced lesion cross-sectional area (postmortem histology). IGF-1 increased plaque's fibrous cap thickness, and reduced necrotic core, macrophage content, and cell apoptosis, consistent with promotion of a stable plaque phenotype. IGF-1 reduced circulating triglycerides, markers of systemic oxidative stress, and CXCL12 chemokine levels. We used spatial transcriptomics (ST) to identify global transcriptome changes in advanced plaque compartments and to obtain mechanistic insights into IGF-1 effects. ST analysis showed that IGF-1 suppressed FOS/FOSB factors and gene expression of MMP9 and CXCL14 in plaque macrophages, suggesting possible involvement of these molecules in IGF-1's effect on atherosclerosis. Thus, IGF-1 reduced coronary plaque burden and promoted features of stable plaque in a pig model, providing support for consideration of clinical trials.
Keywords: Atherosclerosis; Cardiology; Growth factors; Plaque formation; Vascular Biology.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

