Complete Tolerogenic Adjuvant Stimulates Regulatory T Cell Response to Immunization
- PMID: 36602931
- PMCID: PMC9998350
- DOI: 10.4049/jimmunol.2200463
Complete Tolerogenic Adjuvant Stimulates Regulatory T Cell Response to Immunization
Abstract
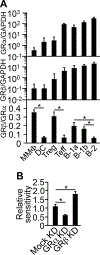
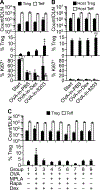
We have determined in mice the minimum composition required for forming a vaccine adjuvant that stimulates a regulatory T (Treg) cell response to immunization, and we named the adjuvant "complete tolerogenic adjuvant." This new kind of adjuvant may let us use the well-proven "Ag with adjuvant" form of immunization for inducing Treg cell-mediated Ag-specific immunosuppression. The minimum composition consists of dexamethasone, rapamycin, and monophosphoryl lipid A at a mass ratio of 8:20:3. By dissecting the respective role of each of these components during immunization, we have further shown why immunosuppressive and immunogenic agents are both needed for forming true adjuvants for Treg cells. This finding may guide the design of additional, and potentially more potent, complete tolerogenic adjuvants with which we may form numerous novel vaccines for treating immune diseases.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures







References
-
- Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, and Ohkura N. 2020. Regulatory T Cells and Human Disease. Annu Rev Immunol 38: 541–566. - PubMed
-
- Chen X, Murakami T, Oppenheim JJ, and Howard OM. 2004. Differential response of murine CD4+CD25+ and CD4+CD25− T cells to dexamethasone-induced cell death. Eur J Immunol 34: 859–869. - PubMed
-
- Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, and Howard OM. 2006. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol 36: 2139–2149. - PubMed
-
- Northrup L, Sullivan BP, Hartwell BL, Garza A, and Berkland C. 2017. Screening Immunomodulators To Skew the Antigen-Specific Autoimmune Response. Mol Pharm 14: 66–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

