The molecular athlete: exercise physiology from mechanisms to medals
- PMID: 36603158
- PMCID: PMC10110736
- DOI: 10.1152/physrev.00017.2022
The molecular athlete: exercise physiology from mechanisms to medals
Abstract
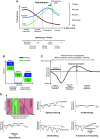
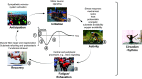
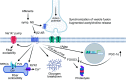
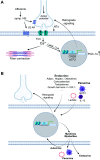
Human skeletal muscle demonstrates remarkable plasticity, adapting to numerous external stimuli including the habitual level of contractile loading. Accordingly, muscle function and exercise capacity encompass a broad spectrum, from inactive individuals with low levels of endurance and strength to elite athletes who produce prodigious performances underpinned by pleiotropic training-induced muscular adaptations. Our current understanding of the signal integration, interpretation, and output coordination of the cellular and molecular mechanisms that govern muscle plasticity across this continuum is incomplete. As such, training methods and their application to elite athletes largely rely on a "trial-and-error" approach, with the experience and practices of successful coaches and athletes often providing the bases for "post hoc" scientific enquiry and research. This review provides a synopsis of the morphological and functional changes along with the molecular mechanisms underlying exercise adaptation to endurance- and resistance-based training. These traits are placed in the context of innate genetic and interindividual differences in exercise capacity and performance, with special consideration given to aging athletes. Collectively, we provide a comprehensive overview of skeletal muscle plasticity in response to different modes of exercise and how such adaptations translate from "molecules to medals."
Keywords: athlete; endurance training; exercise; resistance training; skeletal muscle.
Conflict of interest statement
C.H. is an associate editor of
Figures













References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

