Evaluation and clinical validation of monkeypox (mpox) virus real-time PCR assays
- PMID: 36603329
- PMCID: PMC9783225
- DOI: 10.1016/j.jcv.2022.105373
Evaluation and clinical validation of monkeypox (mpox) virus real-time PCR assays
Abstract
Background: In spring of 2022, an outbreak of monkeypox (mpox) spread worldwide. Here, we describe performance characteristics of monkeypox virus (MPXV)-specific and pan-orthopoxvirus qPCR assays for clinical use.
Methods: We validated probe-based qPCR assays targeting MPXV-specific loci F3L and G2R (genes MPXVgp052/OPG065 and MPXVgp002 and gp190/OPG002, respectively) and a pan-orthopoxvirus assay targeting the E9L locus (MPXVgp057/OPG071). Clinical samples and synthetic controls were extracted using the Roche MP96 or Promega Maxwell 48 instrument. qPCR was performed on the AB7500 thermocycler. Synthetic control DNA and high concentration clinical samples were quantified by droplet PCR. Cross-reactivity was evaluated for camelpox and cowpox genomic DNA, vaccinia culture supernatant, and HSV- and VZV-positive clinical specimens. We also tested the performance of the F3L assay using dry swabs, Aptima vaginal and rectal swabs, nasopharyngeal, rectal, and oral swabs, cerebrospinal fluid, plasma, serum, whole blood, breastmilk, urine, saliva, and semen.
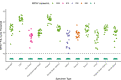
Results: The MPXV-F3L assay is reproducible at a limit of detection (LoD) of 65.6 copies/mL of viral DNA in viral transport medium/universal transport medium (VTM/UTM), or 3.3 copies/PCR reaction. No cross-reactivity with herpesviruses or other poxviruses was observed. MPXV-F3L detects MPXV DNA in alternative specimen types, with an LoD ranging between 260-1000 copies/mL, or 5.7-10 copies/PCR reaction. In clinical swab VTM specimens, MPXV-F3L and MPXV-G2R assays outperformed OPXV-E9L by an average of 2.4 and 2.8 Cts, respectively. MPXV-G2R outperformed MPXV-F3L by 0.4 Cts, consistent with presence of two copies of G2R present in labile inverted terminal repeats (ITRs) of MPXV genome.
Conclusions: MPXV is readily detected by qPCR using three clinically validated assays.
Keywords: Alternative specimen; F3L; G2R; Monkeypox virus; Orthopox; mpox; qPCR.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ALG reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen and Hologic and research support from Gilead and Merck, outside of the described work. Dr Kachikis reported serving as a research consultant for Pfizer and GlaxoSmithKline on maternal immunization-related projects in 2020 and as an unpaid consultant for GlaxoSmithKline in 2022 outside the submitted work. Dr Kachikis reported receiving grant support from Merck and Pfizer outside the submitted work.
Figures

References
-
- Berthet N, Descorps-Declère S, Besombes C, Curaudeau M, Nkili Meyong AA, Selekon B, Labouba I, Gonofio EC, Ouilibona RS, Simo Tchetgna HD, Feher M, Fontanet A, Kazanji M, Manuguerra J-C, Hassanin A, Gessain A, Nakoune E. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. 1. Sci. Rep. 2021;11 - PMC - PubMed
-
- Thomassen HA, Fuller T, Asefi-Najafabady S, Shiplacoff JAG, Mulembakani PM, Blumberg S, Johnston SC, Kisalu NK, Kinkela TL, Fair JN, Wolfe ND, Shongo RL, LeBreton M, Meyer H, Wright LL, Muyembe J-J, Buermann W, Okitolonda E, Hensley LE, Lloyd-Smith JO, Smith TB, Rimoin AW. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. PLoS ONE. 2013;8 - PMC - PubMed
-
- Nolen LD, Osadebe L, Katomba J, Likofata J, Mukadi D, Monroe B, Doty J, Hughes CM, Kabamba J, Malekani J, Bomponda PL, Lokota JI, Balilo MP, Likafi T, Lushima RS, Ilunga BK, Nkawa F, Pukuta E, Karhemere S, Tamfum J-JM, Nguete B, Wemakoy EO, McCollum AM, Reynolds MG. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016;22:1014–1021. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

