Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune-tolerant phase
- PMID: 36603573
- PMCID: PMC10121308
- DOI: 10.3350/cmh.2022.0322
Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune-tolerant phase
Abstract
Background/aims: The histologic status of the immune-tolerant (IT) phase of chronic hepatitis B relative to long-term outcomes is unclear. This study aimed to discover how the serological criteria currently in use correspond to histologic criteria in determining the IT phase and indication for liver biopsy.
Methods: Patients in the serological IT phase determined by positive hepatitis B e antigen, hepatitis B virus (HBV) DNA ≥106 IU/mL, and normal or minimally elevated alanine aminotransferase (ALT) ≤60 IU/L, who underwent liver biopsy at three different hospitals were included. The distribution of the histologic IT phase, defined as fibrosis of stage 1 or less and inflammation of grade 1 or less, was compared with that of the serological IT phase. The risk factors for the incidence of liver-related events, such as hepatocellular carcinoma, liver cirrhosis, liver transplantation, and death, were also analyzed.
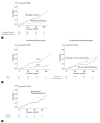
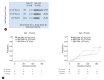
Results: Eighty-two (31.7%) out of 259 clinically suspected IT phase patients belonged to the histologic IT phase. Age over 35, high AST, and low albumin were useful for ruling out the histologic IT phase. Risk factors predicting liver-related events were age and significant fibrosis stage. There was no significant difference in the proportion of histologic IT phase and clinical prognosis between normal ALT and mildly elevated ALT groups. However, even in patients with normal ALT, age was an important factor in predicting the presence of the histologic IT phase.
Conclusion: A significant number of patients who belonged to the serological IT phase were not in the histologic IT phase. Patients over 35 years and those with high AST, low albumin, and low HBV DNA levels were more likely to experience poor long-term clinical outcomes. Therefore, additional histologic assessment should be considered.
Keywords: Biopsy; Fibrosis; Hepatitis B virus.
Conflict of interest statement
The authors have no conflictsto disclose.
Figures

Comment in
-
Letter regarding "Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune-tolerant phase".Clin Mol Hepatol. 2023 Apr;29(2):510-512. doi: 10.3350/cmh.2023.0018. Epub 2023 Jan 30. Clin Mol Hepatol. 2023. PMID: 36710605 Free PMC article. No abstract available.
-
Correspondence on Letter regarding "Long-term prognosis and the need for histologic assessment of chronic hepatitis B in the serological immune tolerant phase".Clin Mol Hepatol. 2023 Apr;29(2):513-515. doi: 10.3350/cmh.2023.0044. Epub 2023 Feb 15. Clin Mol Hepatol. 2023. PMID: 36788758 Free PMC article. No abstract available.
-
The imitator of immune-tolerant chronic hepatitis B: A killer in disguise.Clin Mol Hepatol. 2023 Apr;29(2):363-366. doi: 10.3350/cmh.2023.0079. Epub 2023 Mar 9. Clin Mol Hepatol. 2023. PMID: 36907572 Free PMC article. No abstract available.
-
Is liver biopsy essential to identifying the immune tolerant phase of chronic hepatitis B?Clin Mol Hepatol. 2023 Apr;29(2):367-370. doi: 10.3350/cmh.2023.0054. Epub 2023 Mar 20. Clin Mol Hepatol. 2023. PMID: 36935649 Free PMC article. No abstract available.
References
-
- European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

