Measurements of Hydroxyl Radical Concentrations during Indoor Cooking Events: Evidence of an Unmeasured Photolytic Source of Radicals
- PMID: 36603843
- PMCID: PMC9850917
- DOI: 10.1021/acs.est.2c05756
Measurements of Hydroxyl Radical Concentrations during Indoor Cooking Events: Evidence of an Unmeasured Photolytic Source of Radicals
Abstract
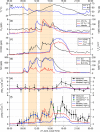
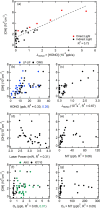
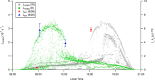
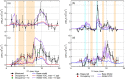
The hydroxyl radical (OH) is the dominant oxidant in the outdoor environment, controlling the lifetimes of volatile organic compounds (VOCs) and contributing to the growth of secondary organic aerosols. Despite its importance outdoors, there have been relatively few measurements of the OH radical in indoor environments. During the House Observations of Microbial and Environmental Chemistry (HOMEChem) campaign, elevated concentrations of OH were observed near a window during cooking events, in addition to elevated mixing ratios of nitrous acid (HONO), VOCs, and nitrogen oxides (NOX). Particularly high concentrations were measured during the preparation of a traditional American Thanksgiving dinner, which required the use of a gas stove and oven almost continually for 6 h. A zero-dimensional chemical model underpredicted the measured OH concentrations even during periods when direct sunlight illuminated the area near the window, which increases the rate of OH production by photolysis of HONO. Interferences with measurements of nitrogen dioxide (NO2) and ozone (O3) suggest that unmeasured photolytic VOCs were emitted during cooking events. The addition of a VOC that photolyzes to produce peroxy radicals (RO2), similar to pyruvic acid, into the model results in better agreement with the OH measurements. These results highlight our incomplete understanding of the nature of oxidation in indoor environments.
Keywords: hydroxyl radical (OH); indoor oxidants; photochemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Griffith S. M.; Hansen R.; Dusanter S.; Michoud V.; Gilman J.; Kuster W.; Veres P.; Graus M.; de Gouw J.; Roberts J.; Young C.; Washenfelder R.; Brown S. S.; Thalman R.; Waxman E.; Volkamer R.; Tsai C.; Stutz J.; Flynn J. H.; Grossberg N.; Lefer B.; Alvarez S. L.; Rappenglueck B.; Mielke L. H.; Osthoff H. D.; Stevens P. S. Measurements of hydroxyl and hydroperoxy radicals during CalNex-LA: Model comparisons and radical budgets. J. Geophys. Res. D: Atmos. 2016, 121, 4211–4232. 10.1002/2015JD024358. - DOI
-
- Ren X.; Harder H.; Martinez M.; Lesher R. L.; Oliger A.; Simpas J. B.; Brune W. H.; Schwab J. J.; Demerjian K. L.; He Y. OH and HO2 chemistry in the urban atmosphere of New York City. Atmos. Environ. 2003, 37, 3639–3651. 10.1016/S1352-2310(03)00459-X. - DOI
-
- Xue L.; Gu R.; Wang T.; Wang X.; Saunders S.; Blake D.; Louie P. K.; Luk C. W.; Simpson I.; Xu Z.; Wang Z.; Gao Y.; Lee S.; Mellouki A.; Wang W. Oxidative capacity and radical chemistry in the polluted atmosphere of Hong Kong and Pearl River Delta region: analysis of a severe photochemical smog episode. Atmos. Chem. Phys. 2016, 16, 9891–9903. 10.5194/acp-16-9891-2016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

