A Unique Role for Protocadherin γC3 in Promoting Dendrite Arborization through an Axin1-Dependent Mechanism
- PMID: 36604170
- PMCID: PMC9908324
- DOI: 10.1523/JNEUROSCI.0729-22.2022
A Unique Role for Protocadherin γC3 in Promoting Dendrite Arborization through an Axin1-Dependent Mechanism
Abstract
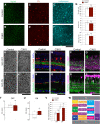
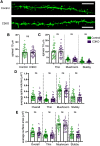
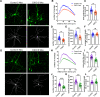
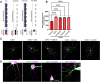
The establishment of a functional cerebral cortex depends on the proper execution of multiple developmental steps, culminating in dendritic and axonal outgrowth and the formation and maturation of synaptic connections. Dysregulation of these processes can result in improper neuronal connectivity, including that associated with various neurodevelopmental disorders. The γ-Protocadherins (γ-Pcdhs), a family of 22 distinct cell adhesion molecules that share a C-terminal cytoplasmic domain, are involved in multiple aspects of neurodevelopment including neuronal survival, dendrite arborization, and synapse development. The extent to which individual γ-Pcdh family members play unique versus common roles remains unclear. We demonstrated previously that the γ-Pcdh-C3 isoform (γC3), via its unique "variable" cytoplasmic domain (VCD), interacts in cultured cells with Axin1, a Wnt-pathway scaffold protein that regulates the differentiation and morphology of neurons. Here, we confirm that γC3 and Axin1 interact in the cortex in vivo and show that both male and female mice specifically lacking γC3 exhibit disrupted Axin1 localization to synaptic fractions, without obvious changes in dendritic spine density or morphology. However, both male and female γC3 knock-out mice exhibit severely decreased dendritic complexity of cortical pyramidal neurons that is not observed in mouse lines lacking several other γ-Pcdh isoforms. Combining knock-out with rescue constructs in cultured cortical neurons pooled from both male and female mice, we show that γC3 promotes dendritic arborization through an Axin1-dependent mechanism mediated through its VCD. Together, these data identify a novel mechanism through which γC3 uniquely regulates the formation of cortical circuitry.SIGNIFICANCE STATEMENT The complexity of a neuron's dendritic arbor is critical for its function. We showed previously that the γ-Protocadherin (γ-Pcdh) family of 22 cell adhesion molecules promotes arborization during development; it remained unclear whether individual family members played unique roles. Here, we show that one γ-Pcdh isoform, γC3, interacts in the brain with Axin1, a scaffolding protein known to influence dendrite development. A CRISPR/Cas9-generated mutant mouse line lacking γC3 (but not lines lacking other γ-Pcdhs) exhibits severely reduced dendritic complexity of cerebral cortex neurons. Using cultured γC3 knock-out neurons and a variety of rescue constructs, we confirm that the γC3 cytoplasmic domain promotes arborization through an Axin1-dependent mechanism. Thus, γ-Pcdh isoforms are not interchangeable, but rather can play unique neurodevelopmental roles.
Keywords: cell adhesion; dendritic arborization; signaling; synapse development; synaptic maturation.
Copyright © 2023 the authors.
Figures






Similar articles
-
A C-terminal motif containing a PKC phosphorylation site regulates γ-Protocadherin-mediated dendrite arborization in the cerebral cortex in vivo.Dev Neurobiol. 2024 Jul;84(3):217-235. doi: 10.1002/dneu.22950. Epub 2024 Jun 4. Dev Neurobiol. 2024. PMID: 38837880 Free PMC article.
-
Protein Kinase C Phosphorylation of a γ-Protocadherin C-terminal Lipid Binding Domain Regulates Focal Adhesion Kinase Inhibition and Dendrite Arborization.J Biol Chem. 2015 Aug 21;290(34):20674-20686. doi: 10.1074/jbc.M115.642306. Epub 2015 Jul 2. J Biol Chem. 2015. PMID: 26139604 Free PMC article.
-
The γ-Protocadherin-C3 isoform inhibits canonical Wnt signalling by binding to and stabilizing Axin1 at the membrane.Sci Rep. 2016 Aug 17;6:31665. doi: 10.1038/srep31665. Sci Rep. 2016. PMID: 27530555 Free PMC article.
-
Protocadherins branch out: Multiple roles in dendrite development.Cell Adh Migr. 2015;9(3):214-26. doi: 10.1080/19336918.2014.1000069. Epub 2015 Apr 14. Cell Adh Migr. 2015. PMID: 25869446 Free PMC article. Review.
-
Regulation of neural circuit formation by protocadherins.Cell Mol Life Sci. 2017 Nov;74(22):4133-4157. doi: 10.1007/s00018-017-2572-3. Epub 2017 Jun 19. Cell Mol Life Sci. 2017. PMID: 28631008 Free PMC article. Review.
Cited by
-
The clustered gamma protocadherin PcdhγC4 isoform regulates cortical interneuron programmed cell death in the mouse cortex.Proc Natl Acad Sci U S A. 2024 Feb 6;121(6):e2313596120. doi: 10.1073/pnas.2313596120. Epub 2024 Jan 29. Proc Natl Acad Sci U S A. 2024. PMID: 38285948 Free PMC article.
-
Protocadherin γC4 Promotes Neuronal Survival in the Mouse Retina Through Its Variable Cytoplasmic Domain.Mol Neurobiol. 2025 Jul 31. doi: 10.1007/s12035-025-05239-z. Online ahead of print. Mol Neurobiol. 2025. PMID: 40742403
-
Surface delivery quantification reveals distinct trafficking efficiencies among clustered protocadherin isoforms.bioRxiv [Preprint]. 2025 Jun 6:2024.09.23.614616. doi: 10.1101/2024.09.23.614616. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2514178122. doi: 10.1073/pnas.2514178122. PMID: 40502045 Free PMC article. Updated. Preprint.
-
Reduced neuronal self-avoidance in mouse starburst amacrine cells with only one Pcdhg isoform.bioRxiv [Preprint]. 2025 May 30:2025.05.29.656828. doi: 10.1101/2025.05.29.656828. bioRxiv. 2025. PMID: 40502005 Free PMC article. Preprint.
-
Visualization of trans-interactions of a protocadherin-α between processes originating from single neurons.iScience. 2023 Jul 17;26(7):107238. doi: 10.1016/j.isci.2023.107238. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534169 Free PMC article.
References
-
- Carriere CH, Wang WX, Sing AD, Fekete A, Jones BE, Yee Y, Ellegood J, Maganti H, Awofala L, Marocha J, Aziz A, Wang LY, Lerch JP, Lefebvre JL (2020) The gamma-protocadherins regulate the survival of GABAergic interneurons during developmental cell death. J Neurosci 40:8652–8668. 10.1523/JNEUROSCI.1636-20.2020 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
