Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury
- PMID: 36604419
- PMCID: PMC9816314
- DOI: 10.1038/s41467-022-35524-7
Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury
Abstract
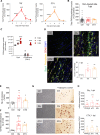
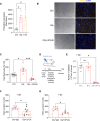
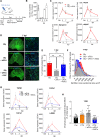
Fibro-adipogenic progenitors (FAPs) play a crucial role in skeletal muscle regeneration, as they generate a favorable niche that allows satellite cells to perform efficient muscle regeneration. After muscle injury, FAP content increases rapidly within the injured muscle, the origin of which has been attributed to their proliferation within the muscle itself. However, recent single-cell RNAseq approaches have revealed phenotype and functional heterogeneity in FAPs, raising the question of how this differentiation of regenerative subtypes occurs. Here we report that FAP-like cells residing in subcutaneous adipose tissue (ScAT), the adipose stromal cells (ASCs), are rapidly released from ScAT in response to muscle injury. Additionally, we find that released ASCs infiltrate the damaged muscle, via a platelet-dependent mechanism and thus contribute to the FAP heterogeneity. Moreover, we show that either blocking ASCs infiltration or removing ASCs tissue source impair muscle regeneration. Collectively, our data reveal that ScAT is an unsuspected physiological reservoir of regenerative cells that support skeletal muscle regeneration, underlining a beneficial relationship between muscle and fat.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Adipose tissue boosts muscle regeneration by supplying mesenchymal stromal cells.Nat Rev Endocrinol. 2023 Jun;19(6):317-318. doi: 10.1038/s41574-023-00831-6. Nat Rev Endocrinol. 2023. PMID: 36991175 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

