Neoantigens: promising targets for cancer therapy
- PMID: 36604431
- PMCID: PMC9816309
- DOI: 10.1038/s41392-022-01270-x
Neoantigens: promising targets for cancer therapy
Abstract
Recent advances in neoantigen research have accelerated the development and regulatory approval of tumor immunotherapies, including cancer vaccines, adoptive cell therapy and antibody-based therapies, especially for solid tumors. Neoantigens are newly formed antigens generated by tumor cells as a result of various tumor-specific alterations, such as genomic mutation, dysregulated RNA splicing, disordered post-translational modification, and integrated viral open reading frames. Neoantigens are recognized as non-self and trigger an immune response that is not subject to central and peripheral tolerance. The quick identification and prediction of tumor-specific neoantigens have been made possible by the advanced development of next-generation sequencing and bioinformatic technologies. Compared to tumor-associated antigens, the highly immunogenic and tumor-specific neoantigens provide emerging targets for personalized cancer immunotherapies, and serve as prospective predictors for tumor survival prognosis and immune checkpoint blockade responses. The development of cancer therapies will be aided by understanding the mechanism underlying neoantigen-induced anti-tumor immune response and by streamlining the process of neoantigen-based immunotherapies. This review provides an overview on the identification and characterization of neoantigens and outlines the clinical applications of prospective immunotherapeutic strategies based on neoantigens. We also explore their current status, inherent challenges, and clinical translation potential.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no potential conflicts of interest. C.H. is the editorial board member of Signal Transduction and Targeted Therapy, but he has not been involved in the process of the manuscript handling.
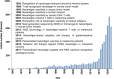
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

