Molecular versatility during pluripotency progression
- PMID: 36604434
- PMCID: PMC9814743
- DOI: 10.1038/s41467-022-35775-4
Molecular versatility during pluripotency progression
Abstract
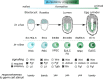
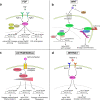
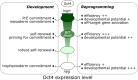
A challenge during development is to ensure lineage segregation while preserving plasticity. Using pluripotency progression as a paradigm, we review how developmental transitions are coordinated by redeployments, rather than global resettings, of cellular components. We highlight how changes in response to extrinsic cues (FGF, WNT, Activin/Nodal, Netrin-1), context- and stoichiometry-dependent action of transcription factors (Oct4, Nanog) and reconfigurations of epigenetic regulators (enhancers, promoters, TrxG, PRC) may confer robustness to naïve to primed pluripotency transition. We propose the notion of Molecular Versatility to regroup mechanisms by which molecules are repurposed to exert different, sometimes opposite, functions in close stem cell configurations.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

