Third-order motifs are sufficient to fully and uniquely characterize spatiotemporal neural network activity
- PMID: 36604489
- PMCID: PMC9816122
- DOI: 10.1038/s41598-022-27188-6
Third-order motifs are sufficient to fully and uniquely characterize spatiotemporal neural network activity
Abstract
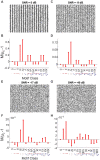
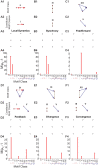
Neuroscientific analyses balance between capturing the brain's complexity and expressing that complexity in meaningful and understandable ways. Here we present a novel approach that fully characterizes neural network activity and does so by uniquely transforming raw signals into easily interpretable and biologically relevant metrics of network behavior. We first prove that third-order (triple) correlation describes network activity in its entirety using the triple correlation uniqueness theorem. Triple correlation quantifies the relationships among three events separated by spatial and temporal lags, which are triplet motifs. Classifying these motifs by their event sequencing leads to fourteen qualitatively distinct motif classes that embody well-studied network behaviors including synchrony, feedback, feedforward, convergence, and divergence. Within these motif classes, the summed triple correlations provide novel metrics of network behavior, as well as being inclusive of commonly used analyses. We demonstrate the power of this approach on a range of networks with increasingly obscured signals, from ideal noiseless simulations to noisy experimental data. This approach can be easily applied to any recording modality, so existing neural datasets are ripe for reanalysis. Triple correlation is an accessible signal processing tool with a solid theoretical foundation capable of revealing previously elusive information within recordings of neural networks.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

