Single-cell-led drug repurposing for Alzheimer's disease
- PMID: 36604493
- PMCID: PMC9816180
- DOI: 10.1038/s41598-023-27420-x
Single-cell-led drug repurposing for Alzheimer's disease
Abstract
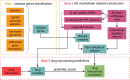
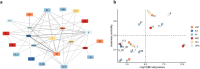
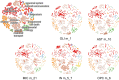
Alzheimer's disease is the most common form of dementia. Notwithstanding the huge investments in drug development, only one disease-modifying treatment has been recently approved. Here we present a single-cell-led systems biology pipeline for the identification of drug repurposing candidates. Using single-cell RNA sequencing data of brain tissues from patients with Alzheimer's disease, genome-wide association study results, and multiple gene annotation resources, we built a multi-cellular Alzheimer's disease molecular network that we leveraged for gaining cell-specific insights into Alzheimer's disease pathophysiology and for the identification of drug repurposing candidates. Our computational approach pointed out 54 candidate drugs, mainly targeting MAPK and IGF1R signaling pathways, which could be further evaluated for their potential as Alzheimer's disease therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

