Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response
- PMID: 36604538
- PMCID: PMC9873558
- DOI: 10.1038/s41591-022-02112-7
Pharmacotypes across the genomic landscape of pediatric acute lymphoblastic leukemia and impact on treatment response
Abstract
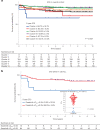
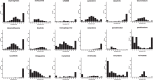
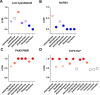
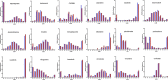
Contemporary chemotherapy for childhood acute lymphoblastic leukemia (ALL) is risk-adapted based on clinical features, leukemia genomics and minimal residual disease (MRD); however, the pharmacological basis of these prognostic variables remains unclear. Analyzing samples from 805 children with newly diagnosed ALL from three consecutive clinical trials, we determined the ex vivo sensitivity of primary leukemia cells to 18 therapeutic agents across 23 molecular subtypes defined by leukemia genomics. There was wide variability in drug response, with favorable ALL subtypes exhibiting the greatest sensitivity to L-asparaginase and glucocorticoids. Leukemia sensitivity to these two agents was highly associated with MRD although with distinct patterns and only in B cell ALL. We identified six patient clusters based on ALL pharmacotypes, which were associated with event-free survival, even after adjusting for MRD. Pharmacotyping identified a T cell ALL subset with a poor prognosis that was sensitive to targeted agents, pointing to alternative therapeutic strategies. Our study comprehensively described the pharmacological heterogeneity of ALL, highlighting opportunities for further individualizing therapy for this most common childhood cancer.
© 2023. The Author(s).
Conflict of interest statement
W.E.E. reports serving as a member of the scientific advisory board for Princess Maxima Centre for Childhood Cancer and serving as a board member for BioSkryb Genomics, neither of which pertain to the submitted work. C.G.M. reports receiving grants from Pfizer and AbbVie and personal fees from Amgen and Illumina outside the submitted work. J.J.Y. reports receiving research funding from Takeda Pharmaceutical Company outside the submitted work and having a patent pending for Methods for Determining Benefit of Chemotherapy. The other authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

