Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis
- PMID: 36604547
- PMCID: PMC10636755
- DOI: 10.1038/s41590-022-01388-8
Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis
Abstract
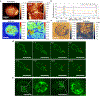
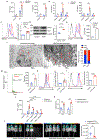
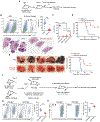
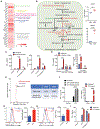
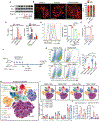
Metastasis is the leading cause of cancer-related deaths and myeloid cells are critical in the metastatic microenvironment. Here, we explore the implications of reprogramming pre-metastatic niche myeloid cells by inducing trained immunity with whole beta-glucan particle (WGP). WGP-trained macrophages had increased responsiveness not only to lipopolysaccharide but also to tumor-derived factors. WGP in vivo treatment led to a trained immunity phenotype in lung interstitial macrophages, resulting in inhibition of tumor metastasis and survival prolongation in multiple mouse models of metastasis. WGP-induced trained immunity is mediated by the metabolite sphingosine-1-phosphate. Adoptive transfer of WGP-trained bone marrow-derived macrophages reduced tumor lung metastasis. Blockade of sphingosine-1-phosphate synthesis and mitochondrial fission abrogated WGP-induced trained immunity and its inhibition of lung metastases. WGP also induced trained immunity in human monocytes, resulting in antitumor activity. Our study identifies the metabolic sphingolipid-mitochondrial fission pathway for WGP-induced trained immunity and control over metastasis.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

