Spectrin-beta 2 facilitates the selective accumulation of GABAA receptors at somatodendritic synapses
- PMID: 36604600
- PMCID: PMC9816108
- DOI: 10.1038/s42003-022-04381-x
Spectrin-beta 2 facilitates the selective accumulation of GABAA receptors at somatodendritic synapses
Abstract
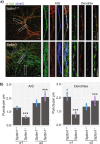
Fast synaptic inhibition is dependent on targeting specific GABAAR subtypes to dendritic and axon initial segment (AIS) synapses. Synaptic GABAARs are typically assembled from α1-3, β and γ subunits. Here, we isolate distinct GABAARs from the brain and interrogate their composition using quantitative proteomics. We show that α2-containing receptors co-assemble with α1 subunits, whereas α1 receptors can form GABAARs with α1 as the sole α subunit. We demonstrate that α1 and α2 subunit-containing receptors co-purify with distinct spectrin isoforms; cytoskeletal proteins that link transmembrane proteins to the cytoskeleton. β2-spectrin was preferentially associated with α1-containing GABAARs at dendritic synapses, while β4-spectrin was associated with α2-containing GABAARs at AIS synapses. Ablating β2-spectrin expression reduced dendritic and AIS synapses containing α1 but increased the number of synapses containing α2, which altered phasic inhibition. Thus, we demonstrate a role for spectrins in the synapse-specific targeting of GABAARs, determining the efficacy of fast neuronal inhibition.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- MH118263/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)
- NS108378/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS101888/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS103865/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS111338/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

