Side effects of Pfizer/BioNTech (BNT162b2) COVID-19 vaccine reported by the Birzeit University community
- PMID: 36604613
- PMCID: PMC9814351
- DOI: 10.1186/s12879-022-07974-3
Side effects of Pfizer/BioNTech (BNT162b2) COVID-19 vaccine reported by the Birzeit University community
Abstract
Background: The Pfizer BioNTech COVID-19 vaccine was the first to receive emergency authorization and approval from the FDA. Therefore, it is preferred by most recipients; however, many people are concerned about the vaccine's side effects. At the time of the study, December 2021, Palestine lacked a national reporting system for monitoring adverse vaccine effects. Therefore, this study investigates the post-vaccine adverse events following the Pfizer/BioNTech COVID-19 Vaccine administration in Palestine and identifies the occurrence, extent, and severity among university staff, employees, and students at Birzeit University.
Method: A questionnaire-based retrospective cross-sectional study was conducted using a university website (Ritaj), social media platforms (e.g., Facebook and Telegram), and in-person interviews. The Chi-square, Fisher's exact, and McNemar's tests were used to investigate significant relationships. Data were analyzed using SPSS version 22.
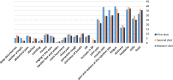
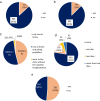
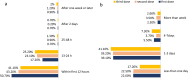
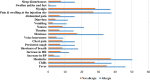
Results: In total, 1137 participants completed the questionnaire, 33.2% were males, and the mean age was 21.163 years. All participants received at least one dose of the Pfizer-BioNTech COVID-19 vaccine. Approximately one-third of participants reported no adverse effects after receiving the first, second, or third doses (34%, 33.6%, and 32.5%, respectively). The most commonly reported adverse events were fever, chills, headache, fatigue, pain and swelling at the injection site, muscle pain, and joint pain. Allergic reactions were reported by 12.7% of the participants; furthermore, participants with a history of allergy or anaphylaxis before vaccination had a significantly higher tendency for post-vaccination allergic reactions. Eight participants reported rare side effects, including 7 (0.6%) cases of thrombocytopenia and one (0.1%) case of myocarditis. Males aged less than 20 years and smokers were significantly less likely to complain of adverse events. The number of reported side effects was significantly higher after the second vaccine dose than after the first dose. Finally, participants infected with COVID-19 before vaccination was significantly associated with side effects such as fever, chills, shortness of breath, and persistent cough.
Conclusion: In this study, the most common post- BNT162b2 Vaccination reported self-limiting side effects similar to those reported by Pfizer/BioNTech Company. However, higher rates of allergic reactions were reported in this sample. Rare side effects, such as thrombocytopenia and myocarditis, were reported by 8 participants. COVID vaccines have been developed at an accelerated pace, and vaccine safety is a top priority; therefore, standard monitoring through a national adverse event reporting system is necessary for safety assurance. Continuous monitoring and long-term studies are required to ensure vaccine safety.
Keywords: Palestine; Pfizer/BioNTech COVID-19 vaccine; Post-vaccination side effects; mRNA COVID-19 vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
-
- Dashboard WCC-. WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. 2022. https://covid19.who.int/.
-
- FDA. Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document Application for licensure of a booster dose for COMIRNATY (COVID-19 Vaccine, mRNA). 2021. https://www.fda.gov/media/152176/download (accessed May 22 2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

