NAMPT encapsulated by extracellular vesicles from young adipose-derived mesenchymal stem cells treated tendinopathy in a "One-Stone-Two-Birds" manner
- PMID: 36604715
- PMCID: PMC9814467
- DOI: 10.1186/s12951-022-01763-5
NAMPT encapsulated by extracellular vesicles from young adipose-derived mesenchymal stem cells treated tendinopathy in a "One-Stone-Two-Birds" manner
Abstract
Background: Tendinopathy is the leading sports-related injury and will cause severe weakness and tenderness. Effective therapy for tendinopathy remains limited, and extracellular vesicles (EVs) derived from adipose tissue-derived mesenchymal stem cells (ADMSCs) have demonstrated great potential in tendinopathy treatment; however, the influence of aging status on EV treatment has not been previously described.
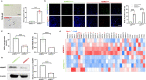
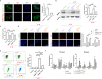
Results: In this study, it was found that ADMSCs derived from old mice (ADMSCold) demonstrated remarkable cellular senescence and impaired NAD+ metabolism compared with ADMSCs derived from young mice (ADMSCyoung). Lower NAMPT contents were detected in both ADMSCold and its secreted EVs (ADMSCold-EVs). Advanced animal experiments demonstrated that ADMSCyoung-EVs, but not ADMSCold-EVs, alleviated the pathological structural, functional and biomechanical properties in tendinopathy mice. Mechanistic analyses demonstrated that ADMSCyoung-EVs improved cell viability and relieved cellular senescence of tenocytes through the NAMPT/SIRT1/PPARγ/PGC-1α pathway. ADMSCyoung-EVs, but not ADMSCold-EVs, promoted phagocytosis and M2 polarization in macrophages through the NAMPT/SIRT1/Nf-κb p65/NLRP3 pathway. The macrophage/tenocyte crosstalk in tendinopathy was influenced by ADMSCyoung-EV treatment and thus it demonstrated "One-Stone-Two-Birds" effects in tendinopathy treatment.
Conclusions: This study demonstrates an effective novel therapy for tendinopathy and uncovers the influence of donor age on curative effects by clarifying the detailed biological mechanism.
Keywords: Adipose tissue-derived mesenchymal stem cells; Extracellular vesicles; Macrophage; NAD+ metabolism; Tendinopathy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Tung KK, Wei JC. Platelet-rich plasma injection vs sham injection and tendon dysfunction in patients with chronic midportion achilles tendinopathy. JAMA. 1975;2021:326. - PubMed
MeSH terms
Substances
Grants and funding
- Grant No.20194Y0385/Project of Shanghai Health Commission
- Grant No.81972444, Grant No.81972095/National Natural Science Foundation of China
- Grant No.81972444, Grant No.81972095/National Natural Science Foundation of China
- Grant No.20S31901400, Grant No.19441901700, Grant No.19441901701, Grant No.19441901702/Project of Shanghai Science and Technology Commission
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

