Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer's disease in individuals with subjective cognitive decline
- PMID: 36604729
- PMCID: PMC9814201
- DOI: 10.1186/s13195-022-01143-z
Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer's disease in individuals with subjective cognitive decline
Abstract
Background: Accessible and cost-effective diagnostic tools are urgently needed to accurately quantify blood biomarkers to support early diagnosis of Alzheimer's disease (AD). In this study, we investigated the ability of plasma amyloid-beta (Aβ)42/Aβ40 ratio measured by an antibody-free mass-spectrometric (MS) method, ABtest-MS, to detect early pathological changes of AD.
Methods: This cohort study included data from the baseline and 2-year follow-up visits from the Fundació ACE Healthy Brain Initiative (FACEHBI) study. Plasma Aβ42/Aβ40 was measured with ABtest-MS and compared to 18F-Florbetaben PET as the reference standard (cutoff for early amyloid deposition of 13.5 centiloids). Cross-validation was performed in an independent DPUK-Korean cohort. Additionally, associations of plasma Aβ42/Aβ40 with episodic memory performance and brain atrophy were assessed.
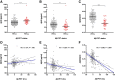
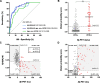
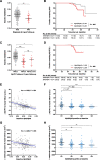
Results: The FACEHBI cohort at baseline included 200 healthy individuals with subjective cognitive decline (SCD), of which 36 (18%) were Aβ-PET positive. Plasma Aβ42/Aβ40 levels were significantly lower in Aβ-PET positive individuals (median [interquartile range, IQR], 0.215 [0.203-0.236]) versus Aβ-PET negative subjects (median [IQR], 0.261 [0.244-0.279]) (P < .001). Plasma Aβ42/Aβ40 was significantly correlated with Aβ-PET levels (rho = -0.390; P < .001) and identified Aβ-PET status with an area under the receiver operating characteristic curve (AUC) of 0.87 (95% confidence interval [CI], 0.80-0.93). A cutoff for the Aβ42/Aβ40 ratio of 0.241 (maximum Youden index) yielded a sensitivity of 86.1% and a specificity of 80.5%. These findings were cross-validated in an independent DPUK-Korean cohort (AUC 0.86 [95% CI 0.77-0.95]). Lower plasma Aβ42/Aβ40 ratio was associated with worse episodic memory performance and increased brain atrophy. Plasma Aβ42/Aβ40 at baseline predicted clinical conversion to mild cognitive impairment and longitudinal changes in amyloid deposition and brain atrophy at 2-year follow-up.
Conclusions: This study suggests that plasma Aβ42/Aβ40, as determined by this MS-based assay, has potential value as an accurate and cost-effective tool to identify individuals in the earliest stages of AD, supporting its implementation in clinical trials, preventative strategies and clinical practice.
Keywords: Alzheimer’s disease; Amyloid; Aβ42/Aβ40; Biomarkers; Blood biomarkers; Mass spectrometry; Plasma; Ratio; Subjective cognitive decline.
© 2023. The Author(s).
Conflict of interest statement
MPL, JAA, LS, NF, SC and JT are full-time employees at Araclon Biotech-Grifols. AR has consulted for Grifols, Prevail Therapeuthics and Landsteiner Genenmed. He reports grants/research funding from Abbvie, Janssen, Grifols and Fundación Bancaria LaCaixa. AR has stocks in Landsteiner Genmed. MB has consulted for Araclon, Avid, Grifols, Lilly, Nutricia, Roche, Eisai and Servier. She received fees from lectures and funds for research from Araclon, Biogen, Grifols, Nutricia, Roche and Servier. She reports grants/research funding from Abbvie, Araclon, Biogen Research Limited, Bioiberica, Grifols, Lilly, S.A, Merck Sharp & Dohme, Kyowa Hakko Kirin, Laboratorios Servier, Nutricia SRL, Oryzon Genomics, Piramal Imaging Limited, Roche Pharma SA, and Schwabe Farma Iberica SLU, all outside the submitted work. She has not received personal compensations from these organizations. JPT, AS, LT, MM, SWS and HJ report no disclosures.
Figures


References
-
- Dementia: World Health Organization. 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 26 July 2022.
-
- Mattsson N, Lönneborg A, Boccardi M, Blennow K, Hansson O. Clinical validity of cerebrospinal fluid Aβ42, tau, and phospho-tau as biomarkers for Alzheimer's disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:196–213. doi: 10.1016/j.neurobiolaging.2016.02.034. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

