Predictors of virologic outcome among people living with HIV who continue a protease inhibitor-based antiretroviral regimen following virologic failure with no or limited resistance
- PMID: 36604746
- PMCID: PMC9814171
- DOI: 10.1186/s12981-022-00494-9
Predictors of virologic outcome among people living with HIV who continue a protease inhibitor-based antiretroviral regimen following virologic failure with no or limited resistance
Abstract
Background: Treatment management after repeated failure of antiretroviral therapy (ART) is difficult due to resistance and adherence challenges. For people who have failed non-nucleoside reverse transcriptase inhibitor-(NNRTI-) and protease inhibitor-(PI-) based regimens with no or limited resistance, remaining on PI-based ART is an option. Using data from an ART strategy trial (A5288) in low/middle-income countries which included this option, we explored whether predictors can be identified distinguishing those who experienced further virologic failure from those who achieved and maintained virologic suppression.
Methods: A5288 enrolled people with confirmed HIV-1 RNA ≥ 1000 copies/mL after ≥ 24 weeks of PI-based ART and prior failure on NNRTI-based ART. This analysis focused on the 278 participants with no resistance to the PI being taken and no or limited nucleoside reverse transcriptase inhibitor (NRTI) resistance, who continued their PI with flexibility to change NRTIs. Proportional hazards models were used to evaluate predictors of virologic failure during follow-up (VF: confirmed HIV-1 RNA ≥ 1000 copies/mL at ≥ 24 weeks of follow-up).
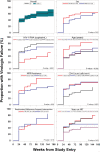
Results: 56% of participants were female. At study entry, median age was 40 years, time on ART 7.8 years, CD4 count 169 cells/mm3, HIV-1 RNA 20,444 copies/mL; and 37% had NRTI resistance. The estimated proportion experiencing VF increased from 39% at week 24 to 60% at week 96. In multivariable analysis, significant predictors at study entry of VF were higher HIV-1 RNA (adjusted hazard ratio: 2.20 for ≥ 10,000 versus < 10,000 copies/mL), lower age (1.96 for < 30 versus ≥ 30 years), NRTI resistance (1.74 for present versus absent), lower CD4 count (1.73 for < 200 versus ≥ 200 cells/mm3), and shorter ART duration (1.62 for < 10 versus ≥ 10 years). There was a strong trend in proportion with VF at week 96 with the number of these five risk factors that a participant had, varying from 8% for zero, to 31%, 40%, 73%, and 100% for one, two, three, and four/five. Only 13% of participants developed new NRTI or PI resistance mutations.
Conclusion: A simple count of five predictors might have value for identifying risk of continued VF. Novel antiretroviral and adherence support interventions are needed to improve virologic outcomes for higher risk individuals.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- World Health Organization. Update of recommendations on first-and second-line antiretroviral regimens. Geneva, Switzerland: World Health Organization; 2019 (WHO/CDS/HIV/19.15). Licence: CC BY-NC-SA 3.0 IGO.https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.1.... Accessed 11 Aug 2020.
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069476/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- P30 MH097488/MH/NIMH NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

