Sickle red blood cell-derived extracellular vesicles activate endothelial cells and enhance sickle red cell adhesion mediated by von Willebrand factor
- PMID: 36604837
- PMCID: PMC10121869
- DOI: 10.1111/bjh.18616
Sickle red blood cell-derived extracellular vesicles activate endothelial cells and enhance sickle red cell adhesion mediated by von Willebrand factor
Abstract
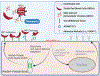
Endothelial activation and sickle red blood cell (RBC) adhesion are central to the pathogenesis of sickle cell disease (SCD). Quantitatively, RBC-derived extracellular vesicles (REVs) are more abundant from SS RBCs compared with healthy RBCs (AA RBCs). Sickle RBC-derived REVs (SS REVs) are known to promote endothelial cell (EC) activation through cell signalling and transcriptional regulation at longer terms. However, the SS REV-mediated short-term non-transcriptional response of EC is unclear. Here, we examined the impact of SS REVs on acute microvascular EC activation and RBC adhesion at 2 h. Compared with AA REVs, SS REVs promoted human pulmonary microvascular ECs (HPMEC) activation indicated by increased von Willebrand factor (VWF) expression. Under microfluidic conditions, we found abnormal SS RBC adhesion to HPMECs exposed to SS REVs. This enhanced SS RBC adhesion was reduced by haeme binding protein haemopexin or VWF cleaving protease ADAMTS13 to a level similar to HPMECs treated with AA REVs. Consistent with these observations, haemin- or SS REV-induced microvascular stasis in SS mice with implanted dorsal skin-fold chambers that was inhibited by ADAMTS13. The adhesion induced by SS REVs was variable and was higher with SS RBCs from patients with increased markers of haemolysis (lactate dehydrogenase and reticulocyte count) or a concomitant clinical diagnosis of deep vein thrombosis. Our results emphasise the critical contribution made by REVs to the pathophysiology of SCD by triggering acute microvascular EC activation and abnormal RBC adhesion. These findings may help to better understand acute pathophysiological mechanism of SCD and thereby the development of new treatment strategies using VWF as a potential target.
Keywords: adamts 13; endothelial inflammation; extracellular vesicles; sickle cell disease; von willebrand factor.
© 2023 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
RA, JAL, UAG, and Case Western Reserve University have financial interests in Hemex Health Inc. JAL, EK, UAG, and Case Western Reserve University have financial interests in BioChip Labs Inc. UAG and Case Western Reserve University have financial interests in Xatek Inc. UAG has financial interests in DxNow Inc. Financial interests include licensed intellectual property, stock ownership, research funding, employment, and consulting. Hemex Health Inc. offers point-of-care diagnostics for hemoglobin disorders, anemia, and malaria. BioChip Labs Inc. offers commercial clinical microfluidic biomarker assays for inherited or acquired blood disorders. Xatek Inc. offers point-of-care global assays to evaluate the hemostatic process. DxNow Inc. offers microfluidic and bio-imaging technologies for in vitro fertilization, forensics, and diagnostics. Competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. GMV and JDB receive research funding for CSL Behring and Astellas/Mitobridge.
Figures


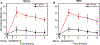

References
-
- Kato Gregory J., Piel Frédéric B., Reid Clarice D., Gaston Marilyn H., Kwaku Ohene-Frempong Lakshmanan Krishnamurti, Smith Wally R., Panepinto Julie A., Weatherall David J., Costa Fernando F., and Vichinsky Elliott P., Sickle cell disease. Nature Reviews Disease Primers, 2018. 4(1): p. 18010. - PubMed
-
- Belcher John D., Chen Chunsheng, Nguyen Julia, Milbauer Liming, Abdulla Fuad, Alayash Abdu I., Smith Ann, Nath Karl A., Hebbel Robert P., and Vercellotti Gregory M., Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood, 2014. 123(3): p. 377–390. - PMC - PubMed
-
- Camus Stephane M., De Moraes Joao A., Bonnin Philippe, Abbyad Paul, Sylvain Le Jeune Francois Lionnet, Loufrani Laurent, Grimaud Linda, Lambry Jean-Christophe, Charue Dominique, Kiger Laurent, Renard Jean-Marie, Larroque Claire, Herve Le Clesiau Alain Tedgui, Bruneval Patrick, Christina Barja-Fidalgo Antigoni Alexandrou, Tharaux Pierre-Louis, Boulanger Chantal M., and Blanc-Brude Olivier P. , Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood, 2015. 125(24): p. 3805–3814. - PMC - PubMed
-
- Camus SM, Gausserès B, Bonnin P, Loufrani L, Grimaud L, Charue D, De Moraes JA, Renard JM, Tedgui A, Boulanger CM, Tharaux PL, and Blanc-Brude OP, Erythrocyte microparticles can induce kidney vaso-occlusions in a murine model of sickle cell disease. Blood, 2012. 120(25): p. 5050–8. - PubMed
-
- Garnier Yohann, Ferdinand Séverine, Garnier Marie, Cita Kizzy-Clara, Hierso Régine, Claes Aurélie, Connes Philippe, Marie-Dominique Hardy-Dessources Claudine Lapouméroulie, Lemonne Nathalie, Maryse Etienne-Julan Wassim El Nemer, and Romana Marc, Plasma microparticles of sickle patients during crisis or taking hydroxyurea modify endothelium inflammatory properties. Blood, 2020. 136(2): p. 247–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

