Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves' Disease
- PMID: 36604959
- PMCID: PMC9816501
- DOI: 10.3803/EnM.2022.1590
Metabolite Changes during the Transition from Hyperthyroidism to Euthyroidism in Patients with Graves' Disease
Abstract
Backgruound: An excess of thyroid hormones in Graves' disease (GD) has profound effects on systemic energy metabolism that are currently partially understood. In this study, we aimed to provide a comprehensive understanding of the metabolite changes that occur when patients with GD transition from hyperthyroidism to euthyroidism with methimazole treatment.
Methods: Eighteen patients (mean age, 38.6±14.7 years; 66.7% female) with newly diagnosed or relapsed GD attending the endocrinology outpatient clinics in a single institution were recruited between January 2019 and July 2020. All subjects were treated with methimazole to achieve euthyroidism. We explored metabolomics by performing liquid chromatography-mass spectrometry analysis of plasma samples of these patients and then performed multivariate statistical analysis of the metabolomics data.
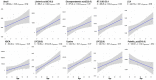
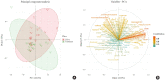
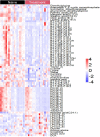
Results: Two hundred metabolites were measured before and after 12 weeks of methimazole treatment in patients with GD. The levels of 61 metabolites, including palmitic acid (C16:0) and oleic acid (C18:1), were elevated in methimazole-naïve patients with GD, and these levels were decreased by methimazole treatment. The levels of another 15 metabolites, including glycine and creatinine, were increased after recovery of euthyroidism upon methimazole treatment in patients with GD. Pathway analysis of metabolomics data showed that hyperthyroidism was closely related to aminoacyl-transfer ribonucleic acid biosynthesis and branched-chain amino acid biosynthesis pathways.
Conclusion: In this study, significant variations of plasma metabolomic patterns that occur during the transition from hyperthyroidism to euthyroidism were detected in patients with GD via untargeted metabolomics analysis.
Keywords: Graves disease; Metabolism; Metabolomics; Thyrotoxicosis.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–4. - PubMed
-
- Greenlund LJ, Nair KS, Brennan MD. Changes in body composition in women following treatment of overt and subclinical hyperthyroidism. Endocr Pract. 2008;14:973–8. - PubMed
-
- Brent GA. Clinical practice: Graves’ disease. N Engl J Med. 2008;358:2594–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

