Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: Findings from population-based cohorts
- PMID: 36604970
- PMCID: PMC9891989
- DOI: 10.1002/jcsm.13160
Recent sarcopenia definitions-prevalence, agreement and mortality associations among men: Findings from population-based cohorts
Abstract
Background: The 2019 European Working Group on Sarcopenia in Older People (EWGSOP2) and the Sarcopenia Definitions and Outcomes Consortium (SDOC) have recently proposed sarcopenia definitions. However, comparisons of the performance of these approaches in terms of thresholds employed, concordance in individuals and prediction of important health-related outcomes such as death are limited. We addressed this in a large multinational assembly of cohort studies that included information on lean mass, muscle strength, physical performance and health outcomes.
Methods: White men from the Health Aging and Body Composition (Health ABC) Study, Osteoporotic Fractures in Men (MrOS) Study cohorts (Sweden, USA), the Hertfordshire Cohort Study (HCS) and the Sarcopenia and Physical impairment with advancing Age (SarcoPhAge) Study were analysed. Appendicular lean mass (ALM) was ascertained using DXA; muscle strength by grip dynamometry; and usual gait speed over courses of 2.4-6 m. Deaths were recorded and verified. Definitions of sarcopenia were as follows: EWGSOP2 (grip strength <27 kg and ALM index <7.0 kg/m2 ), SDOC (grip strength <35.5 kg and gait speed <0.8 m/s) and Modified SDOC (grip strength <35.5 kg and gait speed <1.0 m/s). Cohen's kappa statistic was used to assess agreement between original definitions (EWGSOP2 and SDOC). Presence versus absence of sarcopenia according to each definition in relation to mortality risk was examined using Cox regression with adjustment for age and weight; estimates were combined across cohorts using random-effects meta-analysis.
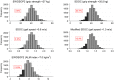
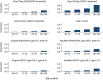
Results: Mean (SD) age of participants (n = 9170) was 74.3 (4.9) years; 5929 participants died during a mean (SD) follow-up of 12.1 (5.5) years. The proportion with sarcopenia according to each definition was EWGSOP2 (1.1%), SDOC (1.7%) and Modified SDOC (5.3%). Agreement was weak between EWGSOP2 and SDOC (κ = 0.17). Pooled hazard ratios (95% CI) for mortality for presence versus absence of each definition were EWGSOP2 [1.76 (1.42, 2.18), I2 : 0.0%]; SDOC [2.75 (2.28, 3.31), I2 : 0.0%]; and Modified SDOC [1.93 (1.54, 2.41), I2 : 58.3%].
Conclusions: There was low prevalence and poor agreement among recent sarcopenia definitions in community-dwelling cohorts of older white men. All indices of sarcopenia were associated with mortality. The strong relationship between sarcopenia and mortality, regardless of the definition, illustrates that identification of appropriate management and lifecourse intervention strategies for this condition is of paramount importance.
Keywords: Ageing; Epidemiology; Mortality; Prevalence; Sarcopenia.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Cyrus Cooper reports personal fees (outside the submitted work) from Amgen, Danone, Eli Lilly, GSK, Kyowa Kirin, Medtronic, Merck, Nestle, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB. Elaine Dennison has received lecture fees and honoraria from UCB, Pfizer, Lilly and Viatris outside of the submitted work. Nicholas Harvey reports consultancy, lecture fees and honoraria (outside the submitted work) from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, UCB, Kyowa Kirin, Consilient Healthcare, Radius Health and Internis Pharma. Roger Fielding reports grants from the National Institutes of Health (National Institute on Aging) and the USDA, during the conduct of the study; grants, personal fees and other from Axcella Health; other from Inside Tracker; grants and personal fees from Biophytis; grants and personal fees from Astellas; personal fees from Pfizer; personal fees from Reneo; personal fees from Cytokinetics; personal fees from Amazentis; grants and personal fees from Nestle; and personal fees from Glaxo Smith Kline, outside the submitted work. Jean‐Yves Reginster declares grant support from industry through institution (IBSA‐Genevrier, Mylan, CNIEL, Radius Health, TRB), lecture fees when speaking at the invitation of sponsor [IBSA‐Genevrier, Mylan, CNIEL, Dairy Research Council (DRC), Nutricia, Danone, Agnovos] and consulting fees or paid advisory boards (IBSA‐Genevrier, Mylan, Radius Health, Pierre Fabre, Faes Pharma, Rejuvenate Biomed, Samumed, Teva, Theramex, Pfizer, Mithra Pharmaceuticals). The remaining authors declare that they have no conflicts of interest.
Figures


References
-
- Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD‐10‐CM) code. J Am Med Dir Assoc 2016;17:675–677. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR000128/TR/NCATS NIH HHS/United States
- MC_UP_A620_1015/MRC_/Medical Research Council/United Kingdom
- MC_PC_21003/MRC_/Medical Research Council/United Kingdom
- R01 NR012459/NR/NINR NIH HHS/United States
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- K07 AG033174/AG/NIA NIH HHS/United States
- U01 AG042168/AG/NIA NIH HHS/United States
- U01 AG042140/AG/NIA NIH HHS/United States
- N01AG62103/NH/NIH HHS/United States
- R01 AG027017/AG/NIA NIH HHS/United States
- G0400491/MRC_/Medical Research Council/United Kingdom
- U01 AG042139/AG/NIA NIH HHS/United States
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- MC_PC_21000/MRC_/Medical Research Council/United Kingdom
- MC_UU_12011/2/MRC_/Medical Research Council/United Kingdom
- UL1 TR002369/TR/NCATS NIH HHS/United States
- R01 AG066671/AG/NIA NIH HHS/United States
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- U01 AG042124/AG/NIA NIH HHS/United States
- MC_PC_21001/MRC_/Medical Research Council/United Kingdom
- U01 AG042145/AG/NIA NIH HHS/United States
- P30 AG024827/AG/NIA NIH HHS/United States
- 19583/VAC_/Versus Arthritis/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
- N01AG62106/NH/NIH HHS/United States
- U01 AG027810/AG/NIA NIH HHS/United States
- G0601019/MRC_/Medical Research Council/United Kingdom
- U01AG042168/AG/NIA NIH HHS/United States
- MC_PC_21022/MRC_/Medical Research Council/United Kingdom
- MR/P020941/1/MRC_/Medical Research Council/United Kingdom
- R01 AG028050/AG/NIA NIH HHS/United States
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- U01 AG042143/AG/NIA NIH HHS/United States
- T32 AG021885/AG/NIA NIH HHS/United States
- N01AG62101/NH/NIH HHS/United States
- U01 AR066160/AR/NIAMS NIH HHS/United States

