RH5.1-CyRPA-Ripr antigen combination vaccine shows little improvement over RH5.1 in a preclinical setting
- PMID: 36605129
- PMCID: PMC9807911
- DOI: 10.3389/fcimb.2022.1049065
RH5.1-CyRPA-Ripr antigen combination vaccine shows little improvement over RH5.1 in a preclinical setting
Abstract
Background: RH5 is the leading vaccine candidate for the Plasmodium falciparum blood stage and has shown impact on parasite growth in the blood in a human clinical trial. RH5 binds to Ripr and CyRPA at the apical end of the invasive merozoite form, and this complex, designated RCR, is essential for entry into human erythrocytes. RH5 has advanced to human clinical trials, and the impact on parasite growth in the blood was encouraging but modest. This study assessed the potential of a protein-in-adjuvant blood stage malaria vaccine based on a combination of RH5, Ripr and CyRPA to provide improved neutralizing activity against P. falciparum in vitro.
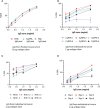
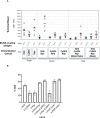
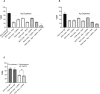
Methods: Mice were immunized with the individual RCR antigens to down select the best performing adjuvant formulation and rats were immunized with the individual RCR antigens to select the correct antigen dose. A second cohort of rats were immunized with single, double and triple antigen combinations to assess immunogenicity and parasite neutralizing activity in growth inhibition assays.
Results: The DPX® platform was identified as the best performing formulation in potentiating P. falciparum inhibitory antibody responses to these antigens. The three antigens derived from RH5, Ripr and CyRPA proteins formulated with DPX induced highly inhibitory parasite neutralising antibodies. Notably, RH5 either as a single antigen or in combination with Ripr and/or CyRPA, induced inhibitory antibodies that outperformed CyRPA, Ripr.
Conclusion: An RCR combination vaccine may not induce substantially improved protective immunity as compared with RH5 as a single immunogen in a clinical setting and leaves the development pathway open for other antigens to be combined with RH5 as a next generation malaria vaccine.
Keywords: CyRPA; Plasmodium falciparum; RH5 complex; Ripr; malaria; merozoite invasion; vaccine.
Copyright © 2022 Healer, Thompson, Mackwell, Browne, Seager, Ngo, Lowes, Silk, Pulido, King, Christen, Noe, Kotraiah, Masendycz, Rajagopalan, Lucas, Stanford, Soisson, Diggs, Miller, Youll, Wycherley, Draper and Cowman.
Conflict of interest statement
The authors LL and RR are employed, and MS was employed by IMV Inc. JC, AN and VK are employed by Leidos Life Sciences. SD is a named inventor on patent applications relating to RH5-based malaria vaccines. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alonso P. L., Sacarlal J., Aponte J. J., Leach A., Macete E., Aide P. (2005). Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366, 2012–2018. doi: 10.1016/S0140-6736(05)67669-6 - DOI - PubMed
-
- Azasi Y., Gallagher S. K., Diouf A., Dabbs R. A., Jin J., Mian S. Y. (2020). Bliss' and loewe's additive and synergistic effects in Plasmodium falciparum growth inhibition by AMA1-RON2L, RH5, RIPR and CyRPA antibody combinations. Sci. Rep. 10, 11802. doi: 10.1038/s41598-020-67877-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

