Oral supplementation with yeast β-glucans improves the resolution of Escherichia coli-associated inflammatory responses independently of monocyte/macrophage immune training
- PMID: 36605196
- PMCID: PMC9809295
- DOI: 10.3389/fimmu.2022.1086413
Oral supplementation with yeast β-glucans improves the resolution of Escherichia coli-associated inflammatory responses independently of monocyte/macrophage immune training
Abstract
Introduction: Confronted with the emerging threat of antimicrobial resistance, the development of alternative strategies to limit the use of antibiotics or potentiate their effect through synergy with the immune system is urgently needed. Many natural or synthetic biological response modifiers have been investigated in this context. Among them, β-glucans, a type of soluble or insoluble polysaccharide composed of a linear or branched string of glucose molecules produced by various cereals, bacteria, algae, and inferior (yeast) and superior fungi (mushrooms) have garnered interest in the scientific community, with not less than 10,000 publications over the last two decades. Various biological activities of β-glucans have been reported, such as anticancer, antidiabetic and immune-modulating effects. In vitro, yeast β-glucans are known to markedly increase cytokine secretion of monocytes/macrophages during a secondary challenge, a phenomenon called immune training.
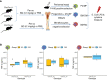
Methods: Here, we orally delivered β-glucans derived from the yeast S. cerevisiae to mice that were further challenged with Escherichia coli.
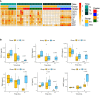
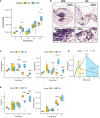
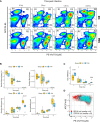
Results: β-glucan supplementation protected the mice from E. coli intraperitoneal and intra-mammary infections, as shown by a lower bacterial burden and greatly diminished tissue damage. Surprisingly, this was not associated with an increased local immune response. In addition, granulocyte recruitment was transient and limited, as well as local cytokine secretion, arguing for faster resolution of the inflammatory response. Furthermore, ex-vivo evaluation of monocytes/macrophages isolated or differentiated from β-glucan-supplemented mice showed these cells to lack a trained response versus those from control mice.
Conclusion: In conclusion, dietary β-glucans can improve the outcome of Escherichia coli infections and dampen tissue damages associated to excessive inflammatory response. The mechanisms associated with such protection are not necessarily linked to immune system hyper-activation or immune training.
Keywords: escherichia coli; immune training; inflammation; innate immunity; macrophages; nutritional immuology; saccharomyces cerevisiae; β-glucans.
Copyright © 2022 Walachowski, Breyne, Secher, Cougoule, Guzylack-Piriou, Meyer, Foucras and Tabouret.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Antimicrobial resistance . Available at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
-
- Centers for Disease Control and Prevention (U.S.) . Antibiotic resistance threats in the united states, 2019. Atlanta, GA, USA: Centers for Disease Control and Prevention (U.S; (2019). Available at: https://stacks.cdc.gov/view/cdc/82532.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

