Potential use of a dried saliva spot (DSS) in therapeutic drug monitoring and disease diagnosis
- PMID: 36605582
- PMCID: PMC9805949
- DOI: 10.1016/j.jpha.2021.11.001
Potential use of a dried saliva spot (DSS) in therapeutic drug monitoring and disease diagnosis
Abstract
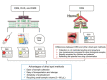
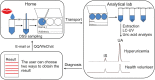
In recent years, scientific researchers have increasingly become interested in noninvasive sampling methods for therapeutic drug monitoring and disease diagnosis. As a result, dried saliva spot (DSS), which is a sampling technique for collecting dried saliva samples, has been widely used as an alternative matrix to serum for the detection of target molecules. Coupling the DSS method with a highly sensitive detection instrument improves the efficiency of the preparation and analysis of biological samples. Furthermore, dried blood spots, dried plasma spots, and dried matrix spots, which are similar to those of the DSS method, are discussed. Compared with alternative biological fluids used in dried spot methods, including serum, tears, urine, and plasma, saliva has the advantage of convenience in terms of sample collection from children or persons with disabilities. This review aims to provide integral strategies and guidelines for dried spot methods to analyze biological samples by illustrating several dried spot methods. Herein, we summarize recent advancements in DSS methods from June 2014 to March 2021 and discuss the advantages and disadvantages of the key aspects of this method, including sample preparation and method validation. Finally, we outline the challenges and prospects of such methods in practical applications.
Keywords: Disease diagnosis; Dried saliva spot; Human saliva; Therapeutic drug monitoring.
© 2021 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Lindon J.C., Holmes E.B., Bollard M.E., et al. Metabonomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9:1–31. - PubMed
-
- Guerra Valero Y.C., Wallis S.C., Lipman J., et al. Clinical application of microsampling versus conventional sampling techniques in the quantitative bioanalysis of antibiotics: A systematic review. Bioanalysis. 2018;10:407–423. - PubMed
-
- Dwivedi J., Namdev K.K., Chilkoti D.C., et al. An improved LC-ESI-MS/MS method to quantify pregabalin in human plasma and dry plasma spot for therapeutic monitoring and pharmacokinetic applications. Ther. Drug Monit. 2018;40:610–619. - PubMed
-
- Neto O.V., Raymundo S., Franzoi M.A., et al. DPD functional tests in plasma, fresh saliva and dried saliva samples as predictors of 5-fluorouracil exposure and occurrence of drug-related severe toxicity. Clin. Biochem. 2018;56:18–25. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

