Robust visual cortex evoked potentials (VEP) in Gnat1 and Gnat2 knockout mice
- PMID: 36605613
- PMCID: PMC9807669
- DOI: 10.3389/fncel.2022.1090037
Robust visual cortex evoked potentials (VEP) in Gnat1 and Gnat2 knockout mice
Abstract
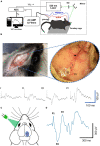
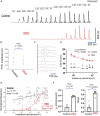
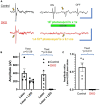
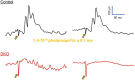
Intrinsically photosensitive retinal ganglion cells (ipRGCs) express the photopigment melanopsin, imparting to themselves the ability to respond to light in the absence of input from rod or cone photoreceptors. Since their discovery ipRGCs have been found to play a significant role in non-image-forming aspects of vision, including circadian photoentrainment, neuroendocrine regulation, and pupillary control. In the past decade it has become increasingly clear that some ipRGCs also contribute directly to pattern-forming vision, the ability to discriminate shapes and objects. However, the degree to which melanopsin-mediated phototransduction, versus that of rods and cones, contributes to this function is still largely unknown. Earlier attempts to quantify this contribution have relied on genetic knockout models that target key phototransductive proteins in rod and cone photoreceptors, ideally to isolate melanopsin-mediated responses. In this study we used the Gnat1-/-; Gnat2cpfl3/cpfl3 mouse model, which have global knockouts for the rod and cone α-transducin proteins. These genetic modifications completely abolish rod and cone photoresponses under light-adapted conditions, locking these cells into a "dark" state. We recorded visually evoked potentials in these animals and found that they still showed robust light responses, albeit with reduced light sensitivity, with similar magnitudes to control mice. These responses had characteristics that were in line with a melanopsin-mediated signal, including delayed kinetics and increased saturability. Additionally, we recorded electroretinograms in a sub-sample of these mice and were unable to find any characteristic waveform related the activation of photoreceptors or second-order retinal neurons, suggesting ipRGCs as the origin of light responses. Our results show a profound ability for melanopsin phototransduction to directly contribute to the primary pattern-forming visual pathway.
Keywords: ipRGCs; melanopsin; phototransduction; primary visual cortex; retina; transducin; vision; visual evoked potential.
Copyright © 2022 Flood, Veloz, Hattar and Carvalho-de-Souza.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Roles of Rods, Cones, and Melanopsin in Photoresponses of M4 Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs) and Optokinetic Visual Behavior.Front Cell Neurosci. 2018 Jul 12;12:203. doi: 10.3389/fncel.2018.00203. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30050414 Free PMC article.
-
C-terminal phosphorylation regulates the kinetics of a subset of melanopsin-mediated behaviors in mice.Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):2741-2746. doi: 10.1073/pnas.1611893114. Epub 2017 Feb 21. Proc Natl Acad Sci U S A. 2017. PMID: 28223508 Free PMC article.
-
Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision.Nature. 2008 May 1;453(7191):102-5. doi: 10.1038/nature06829. Epub 2008 Apr 23. Nature. 2008. PMID: 18432195 Free PMC article.
-
Blurring the boundaries of vision: novel functions of intrinsically photosensitive retinal ganglion cells.J Exp Neurosci. 2013 Sep 3;7:43-50. doi: 10.4137/JEN.S11267. eCollection 2013. J Exp Neurosci. 2013. PMID: 25157207 Free PMC article. Review.
-
Melanopsin phototransduction: beyond canonical cascades.J Exp Biol. 2021 Dec 1;224(23):jeb226522. doi: 10.1242/jeb.226522. Epub 2021 Nov 29. J Exp Biol. 2021. PMID: 34842918 Free PMC article. Review.
Cited by
-
Establishing Functional Retina in a Dish: Progress and Promises of Induced Pluripotent Stem Cell-Based Retinal Neuron Differentiation.Int J Mol Sci. 2023 Sep 4;24(17):13652. doi: 10.3390/ijms241713652. Int J Mol Sci. 2023. PMID: 37686457 Free PMC article. Review.
-
Melanopsin-mediated amplification of cone signals in the human visual cortex.Proc Biol Sci. 2024 May;291(2023):20232708. doi: 10.1098/rspb.2023.2708. Epub 2024 May 29. Proc Biol Sci. 2024. PMID: 38808443 Free PMC article.
-
Retinal inputs that drive optomotor responses of mice under mesopic conditions.IBRO Neurosci Rep. 2024 Jul 21;17:138-144. doi: 10.1016/j.ibneur.2024.07.003. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39170059 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

