Biocatalytic Friedel-Crafts Reactions
- PMID: 36606067
- PMCID: PMC9804301
- DOI: 10.1002/cctc.202200636
Biocatalytic Friedel-Crafts Reactions
Abstract
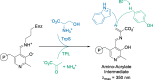
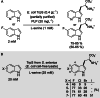
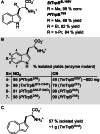
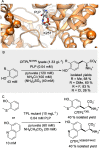
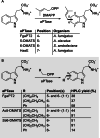
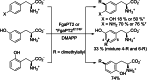
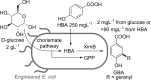
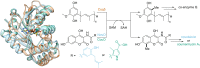
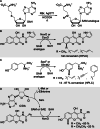
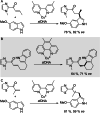
Friedel-Crafts alkylation and acylation reactions are important methodologies in synthetic and industrial chemistry for the construction of aryl-alkyl and aryl-acyl linkages that are ubiquitous in bioactive molecules. Nature also exploits these reactions in many biosynthetic processes. Much work has been done to expand the synthetic application of these enzymes to unnatural substrates through directed evolution. The promise of such biocatalysts is their potential to supersede inefficient and toxic chemical approaches to these reactions, with mild operating conditions - the hallmark of enzymes. Complementary work has created many bio-hybrid Friedel-Crafts catalysts consisting of chemical catalysts anchored into biomolecular scaffolds, which display many of the same desirable characteristics. In this Review, we summarise these efforts, focussing on both mechanistic aspects and synthetic considerations, concluding with an overview of the frontiers of this field and routes towards more efficient and benign Friedel-Crafts reactions for the future of humankind.
Keywords: Acylation; Artificial Enzymes; Biocatalysis; DNA Catalysis; Enzymes; Friedel-Crafts Alkylation.
© 2022 The Authors. ChemCatChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


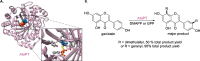





References
-
- Friedel C., Crafts J. M., Comptes rendus des séances 1877, 1392–1395.
-
- None
-
- Heravi M. M., Zadsirjan V., Heydari M., Masoumi B., Chem. Rec. 2019, 19, 2236–2340;
Publication types
LinkOut - more resources
Full Text Sources
