Cancer nanomedicine in preoperative therapeutics: Nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy
- PMID: 36606253
- PMCID: PMC9792706
- DOI: 10.1016/j.bioactmat.2022.12.010
Cancer nanomedicine in preoperative therapeutics: Nanotechnology-enabled neoadjuvant chemotherapy, radiotherapy, immunotherapy, and phototherapy
Abstract
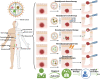
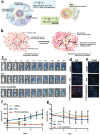
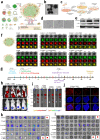
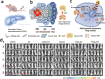
Surgical resection remains a mainstay in the treatment of malignant solid tumors. However, the use of neoadjuvant treatments, including chemotherapy, radiotherapy, phototherapy, and immunotherapy, either alone or in combination, as a preoperative intervention regimen, have attracted increasing attention in the last decade. Early randomized, controlled trials in some tumor settings have not shown a significant difference between the survival rates in long-term neoadjuvant therapy and adjuvant therapy. However, this has not hampered the increasing use of neoadjuvant treatments in clinical practice, due to its evident downstaging of primary tumors to delineate the surgical margin, tailoring systemic therapy response as a clinical tool to optimize subsequent therapeutic regimens, and decreasing the need for surgery, with its potential for increased morbidity. The recent expansion of nanotechnology-based nanomedicine and related medical technologies provides a new approach to address the current challenges of neoadjuvant therapy for preoperative therapeutics. This review not only summarizes how nanomedicine plays an important role in a range of neoadjuvant therapeutic modalities, but also highlights the potential use of nanomedicine as neoadjuvant therapy in preclinical and clinic settings for tumor management.
Keywords: Nanotechnology; Neoadjuvant treatment; Preoperative preparation; Surgical resection.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- King T.A., Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015;12:335–343. - PubMed
-
- Wang C., Fan W., Zhang Z., Wen Y., Xiong L., Chen X. Advanced nanotechnology leading the way to multimodal imaging‐guided precision surgical therapy. Adv. Mater. 2019;31 - PubMed
-
- Byrd D.R., Brierley J.D., Baker T.P., Sullivan D.C., Gress D.M. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA A Cancer J. Clin. 2021;71:140–148. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

