Engineering of immunoinstructive extracellular matrices for enhanced osteoinductivity
- PMID: 36606254
- PMCID: PMC9800268
- DOI: 10.1016/j.bioactmat.2022.12.017
Engineering of immunoinstructive extracellular matrices for enhanced osteoinductivity
Abstract
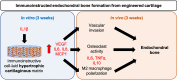
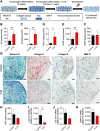
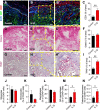
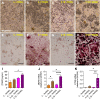
The increasing recognition of the contribution of the immune system to activate and prime regeneration implies that tissue engineering strategies and biomaterials design should target regulation of early immunological processes. We previously proposed the cell-based engineering and devitalization of extracellular matrices (ECMs) as a strategy to generate implant materials delivering custom-defined signals. Here, in the context of bone regeneration, we aimed at enhancing the osteoinductivity of such ECMs by enriching their immunomodulatory factors repertoire. Priming with IL1β a cell line overexpressing BMP-2 enabled engineering of ECMs preserving osteoinductive signals and containing larger amounts of angiogenic (VEGF) and pro-inflammatory molecules (IL6, IL8 and MCP1). Upon implantation, these IL1β-induced materials enhanced processes typical of the inflammatory phase (e.g., vascular invasion, osteoclast recruitment and differentiation), leading to 'regenerative' events (e.g., M2 macrophage polarization) and ultimately resulting in faster and more efficient bone formation. These results bear relevance towards the manufacturing of potent off-the-shelf osteoinductive materials and outline the broader paradigm of engineering immunoinstructive implants to enhance tissue regeneration.
Keywords: Bone tissue engineering; Hypertrophic cartilage matrices; Immunomodulation; Inflammation; Osteoinduction.
© 2022 The Authors.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Rho J., Takami M., Choi Y. Osteoimmunology: interactions of the immune and skeletal systems. Mol. Cell. 2004;17(1):1–9. - PubMed
-
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007;7(4):292–304. - PubMed
-
- Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. - PubMed
-
- Ono T., Takayanagi H. Osteoimmunology in bone fracture healing. Curr. Osteoporos. Rep. 2017;15(4):367–375. - PubMed
LinkOut - more resources
Full Text Sources

