Quantitative magnetic resonance imaging (qMRI) in axial spondyloarthritis
- PMID: 36607267
- PMCID: PMC10078871
- DOI: 10.1259/bjr.20220675
Quantitative magnetic resonance imaging (qMRI) in axial spondyloarthritis
Abstract
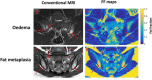
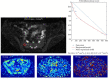
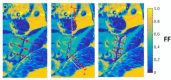
Imaging, and particularly MRI, plays a crucial role in the assessment of inflammation in rheumatic disease, and forms a core component of the diagnostic pathway in axial spondyloarthritis. However, conventional imaging techniques are limited by image contrast being non-specific to inflammation and a reliance on subjective, qualitative reader interpretation. Quantitative MRI methods offer scope to address these limitations and improve our ability to accurately and precisely detect and characterise inflammation, potentially facilitating a more personalised approach to management. Here, we review quantitative MRI methods and emerging quantitative imaging biomarkers for imaging inflammation in axial spondyloarthritis. We discuss the potential benefits as well as the practical considerations that must be addressed in the movement toward clinical translation of quantitative imaging biomarkers.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

