Multimodal brain age estimates relate to Alzheimer disease biomarkers and cognition in early stages: a cross-sectional observational study
- PMID: 36607335
- PMCID: PMC9988262
- DOI: 10.7554/eLife.81869
Multimodal brain age estimates relate to Alzheimer disease biomarkers and cognition in early stages: a cross-sectional observational study
Abstract
Background: Estimates of 'brain-predicted age' quantify apparent brain age compared to normative trajectories of neuroimaging features. The brain age gap (BAG) between predicted and chronological age is elevated in symptomatic Alzheimer disease (AD) but has not been well explored in presymptomatic AD. Prior studies have typically modeled BAG with structural MRI, but more recently other modalities, including functional connectivity (FC) and multimodal MRI, have been explored.
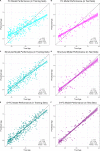
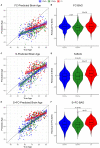
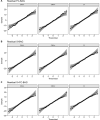
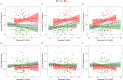
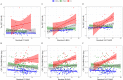
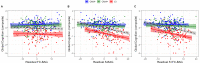
Methods: We trained three models to predict age from FC, structural (S), or multimodal MRI (S+FC) in 390 amyloid-negative cognitively normal (CN/A-) participants (18-89 years old). In independent samples of 144 CN/A-, 154 CN/A+, and 154 cognitively impaired (CI; CDR > 0) participants, we tested relationships between BAG and AD biomarkers of amyloid and tau, as well as a global cognitive composite.
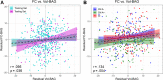
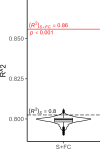
Results: All models predicted age in the control training set, with the multimodal model outperforming the unimodal models. All three BAG estimates were significantly elevated in CI compared to controls. FC-BAG was significantly reduced in CN/A+ participants compared to CN/A-. In CI participants only, elevated S-BAG and S+FC BAG were associated with more advanced AD pathology and lower cognitive performance.
Conclusions: Both FC-BAG and S-BAG are elevated in CI participants. However, FC and structural MRI also capture complementary signals. Specifically, FC-BAG may capture a unique biphasic response to presymptomatic AD pathology, while S-BAG may capture pathological progression and cognitive decline in the symptomatic stage. A multimodal age-prediction model improves sensitivity to healthy age differences.
Funding: This work was supported by the National Institutes of Health (P01-AG026276, P01- AG03991, P30-AG066444, 5-R01-AG052550, 5-R01-AG057680, 1-R01-AG067505, 1S10RR022984-01A1, and U19-AG032438), the BrightFocus Foundation (A2022014F), and the Alzheimer's Association (SG-20-690363-DIAN).
Keywords: Alzheimer disease; brain aging; human; machine learning; medicine; neuroscience; resting-state functional connectivity; structural MRI.
Plain language summary
The brains of people with advanced Alzheimer’s disease often look older than expected based on the patients’ actual age. This ‘brain age gap’ (how old a brain appears compared to the person’s chronological age) can be calculated thanks to machine learning algorithms which analyse images of the organ to detect changes related to aging. Traditionally, these models have relied on images of the brain structure, such as the size and thickness of various brain areas; more recent models have started to use activity data, such as how different brain regions work together to form functional networks. While the brain age gap is a useful measure for researchers who investigate aging and disease, it is not yet helpful for clinicians. For example, it is unclear whether the machine learning algorithm could detect changes in the brains of individuals in the initial stages of Alzheimer’s disease, before they start to manifest cognitive symptoms. Millar et al. explored this question by testing whether models which incorporate structural and activity data could be more sensitive to these early changes. Three machine learning algorithms (relying on either structural data, activity data, or combination of both) were used to predict the brain ages of participants with no sign of disease; with biological markers of Alzheimer’s disease but preserved cognitive functions; and with marked cognitive symptoms of the condition. Overall, the combined model was slightly better at predicting the brain age of healthy volunteers, and all three models indicated that patients with dementia had a brain which looked older than normal. For this group, the model based on structural data was also able to make predictions which reflected the severity of cognitive decline. Crucially, the algorithm which used activity data predicted that, in individuals with biological markers of Alzheimer’s disease but no cognitive impairment, the brain looked in fact younger than chronological age. Exactly why this is the case remains unclear, but this signal may be driven by neural processes which unfold in the early stages of the disease. While more research is needed, the work by Millar et al. helps to explore how various types of machine learning models could one day be used to assess and predict brain health.
© 2023, Millar et al.
Conflict of interest statement
PM, BG, PL, RP, RA, HM, GN, JM, BA No competing interests declared, TB received doses (AV45, AV1451) and partial support for PET scanning through an investigator-initiated research grant awarded to Washington University from Avid Radiopharmaceuticals (a wholly-owned subsidiary of Eli Lilly and Company). The author received consulting fees from Eisai, Siemens, and received payment for Biogen speaker's bureau. Tammie Benzinger acts as site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly and Company, Biogen, Eisai, Jaansen and Roche. The author has no other competing interests to declare, CC has received research support from Biogen, EISAI, Alector and Parabon. Carlos Cruchaga is a member of the advisory board of Vivid Genetics, Circular Genomics and Alector. The author has no other competing interests to declare, AF has received consulting fees from DiamiR and Siemens Healthcare Diagnostics Inc and has received consulting fees for participation on Scientific advisory boards for Roche Diagnostics, Genentech and Diadem. The author has received travel support for in-person attendance at ABC-DS Meeting/Retreat and travel support/honorarium for in-person attendance at Scientific Advisory Board meeting for South Texas Alzheimer's Disease Research Center (ADRC). The author has no other competing interests to declare, JH has received consulting fees from Roche and Parabon Nanolabs. The author has no other competing interests to declare, SS received personal honoraria for presenting lectures from the University of Wisconsin, St. Luke's Hospital, Houston Methodist Medical Center, personal Honoraria for serving on the Alzheimer Disease Center Clinical Task Force from University of Washington and personal honoraria for serving on the National Centralized Repository for Alzheimer's Disease biospecimen review committee from University of Indiana. The author received travel support from National Institute on Aging grant R01AG070941, and is a board member of the Greater Missouri Alzheimer's Association. The author received plasma Ab42/Ab40 data provided by C2N Diagnostics at no cost. No payments/research funding was provided by C2N Diagnostics. No gifts/financial incentives of any kind have been provided to Dr. Schindler by C2N Diagnostics. The author has no other competing interests to declare, GD received fees for consulting and for acting as Dementia Topic Editor from DynaMed (EBSCO Health) and received fees for consulting, grant writing / implementation Parabon Nanonlabs. The author received payment for CME Content development from PeerView Media and Continuing Education Inc, payment for educational content development and focus group participation from Eli Lilly Co, and payment for continuum manuscript authorship from the American Academy of Neurology. The author received payment for expert testimony in the case of Wernicke encephalopathy from Barrow Law. Gregory S Day acts as Clinical Director for Anti-NMDA Receptor Encephalitis Foundation, Inc. The author has stock holdings at ANI Pharmaceuticals, Inc and stock options at Parabon Nanolabs. The author has no other competing interests to declare, MF received grants from AbbVie, Eisai, Novartis, ADCS Posiphen, Genentech and Suven Life Sciences (no grant numbers available). The author has received consulting fees from Artery Therapeutics, Avanir, Biogen, Cyclo Therapeutics, Green Valley, Lexeo, McClena, Nervive, Oligomerix, Pinteon, Prothena, Vaxinity, Athira, AZTherapies, Cognition Therapeutics, Gemvax, Ionis, Longeveron, Merck, Neurotrope Biosciences, Otsuka, Proclara and SToP-AD. The author has no other competing interests to declare, received funding and non-financial support for the DIAN-TU-001 trial from Avid Radiopharmaceuticals, and funding for the DIAN-TU-001 trial from Janssen, Hoffman La-Roche/Genentech, Eli Lilly & Co., Eisai, Biogen, AbbVie and Bristol Meyer Squibb. The author has equity ownership interest in C2N Diagnostics and receives royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. The author received International Conference Lecture Honoraria from Korean Dementia Association and Conference Lecture Honoraria from Weill Cornell Medical College. The author received support for travel expenses from Alzheimer's Association Roundtable and Duke Margolis Alzheimer's Roundtable. The author participates on an unpaid Advisory Board for Roche Gantenerumab Steering Committee and Biogen - Combination Therapy for Alzheimer's Disease, and participates on an unpaid Scientific Advisory Board for UK Dementia Research Institute at University College London and Stanford University, Next Generation Translational Proteomics for Alzheimer's and Related Dementias. The author receives an income from C2N Diagnostics for serving on the scientific advisory board. The author has received equipment and materials from Avid Radiopharmaceuticals, Eli Lilly & Co, Hoffman La-Roche, Eisai and Janssen. Unrelated to this article, Randall Bateman serves as principal investigator of the DIAN-TU, which is supported by the Alzheimer's Association, GHR Foundation, an anonymous organization and the DIAN-TU Pharma Consortium (Active: Eli Lilly and Company/Avid Radiopharmaceuticals, F. Hoffman-La Roche/Genentech, Biogen, Eisai, and Janssen. Previous: Abbvie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, and United Neuroscience). In addition, in-kind support has been received from CogState and Signant Health. Unrelated to this article Randall Bateman has submitted the US nonprovisional patent application "Methods for Measuring the Metabolism of CNS Derived Biomolecules In Vivo" and provisional patent application "Plasma Based Methods for Detecting CNS Amyloid Deposition". The author has no other competing interests to declare, RB has received consulting fees from Barcelona Brain Research Center BBRC and Native Alzheimer Disease-Related Resource Center in Minority Aging Research, Ext Adv Board. The author has received payment or honoraria for lectures from Montefiore Grand Rounds, NY and Tetra-Inst ADRC seminar series, Grand Rds, NY. The author has participated on the Research Strategy Council for the Cure Alzheimer's Fund, the Diverse VCID Observational Study Monitoring Board and the LEADS Advisory Board, Indiana University. The author has no other competing interests to declare
Figures


Update of
- doi: 10.1101/2022.08.25.505251
References
-
- Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychological Monographs. 1946;60:i1–i48. doi: 10.1037/h0093567. - DOI
-
- Bashyam VM, Erus G, Doshi J, Habes M, Nasrallah IM, Truelove-Hill M, Srinivasan D, Mamourian L, Pomponio R, Fan Y, Launer LJ, Masters CL, Maruff P, Zhuo C, Völzke H, Johnson SC, Fripp J, Koutsouleris N, Satterthwaite TD, Wolf D, Gur RE, Gur RC, Morris J, Albert MS, Grabe HJ, Resnick S, Bryan RN, Wolk DA, Shou H, Davatzikos C. Mri signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143:2312–2324. doi: 10.1093/brain/awaa160. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SG-20-690363-DIAN/ALZ/Alzheimer's Association/United States
- U19-AG032438/NH/NIH HHS/United States
- K01 AG053474/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- P01-AG03991/NH/NIH HHS/United States
- S10 RR022984/RR/NCRR NIH HHS/United States
- R01 AG057680/AG/NIA NIH HHS/United States
- P30-AG066444/NH/NIH HHS/United States
- 5-R01-AG052550/NH/NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- R01 NS092865/NS/NINDS NIH HHS/United States
- P30 AG066444/AG/NIA NIH HHS/United States
- R01 AG067505/AG/NIA NIH HHS/United States
- 5-R01-AG057680/NH/NIH HHS/United States
- R01 AG052550/AG/NIA NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- P01-AG026276/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

