Galactic cosmic radiation exposure causes multifaceted neurocognitive impairments
- PMID: 36607431
- PMCID: PMC9823026
- DOI: 10.1007/s00018-022-04666-8
Galactic cosmic radiation exposure causes multifaceted neurocognitive impairments
Abstract
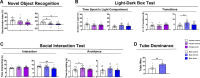
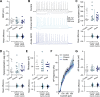
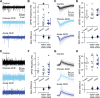
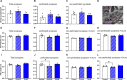
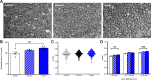
Technological advancements have facilitated the implementation of realistic, terrestrial-based complex 33-beam galactic cosmic radiation simulations (GCR Sim) to now probe central nervous system functionality. This work expands considerably on prior, simplified GCR simulations, yielding new insights into responses of male and female mice exposed to 40-50 cGy acute or chronic radiations relevant to deep space travel. Results of the object in updated location task suggested that exposure to acute or chronic GCR Sim induced persistent impairments in hippocampus-dependent memory formation and reconsolidation in female mice that did not manifest robustly in irradiated male mice. Interestingly, irradiated male mice, but not females, were impaired in novel object recognition and chronically irradiated males exhibited increased aggressive behavior on the tube dominance test. Electrophysiology studies used to evaluate synaptic plasticity in the hippocampal CA1 region revealed significant reductions in long-term potentiation after each irradiation paradigm in both sexes. Interestingly, network-level disruptions did not translate to altered intrinsic electrophysiological properties of CA1 pyramidal cells, whereas acute exposures caused modest drops in excitatory synaptic signaling in males. Ultrastructural analyses of CA1 synapses found smaller postsynaptic densities in larger spines of chronically exposed mice compared to controls and acutely exposed mice. Myelination was also affected by GCR Sim with acutely exposed mice exhibiting an increase in the percent of myelinated axons; however, the myelin sheathes on small calibur (< 0.3 mm) and larger (> 0.5 mm) axons were thinner when compared to controls. Present findings might have been predicted based on previous studies using single and mixed beam exposures and provide further evidence that space-relevant radiation exposures disrupt critical cognitive processes and underlying neuronal network-level plasticity, albeit not to the extent that might have been previously predicted.
Keywords: Cognitive dysfunction; Electrophysiology; Space radiation; Synaptic plasticity.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflict of interest or competing interests to disclose.
Figures


References
-
- Cucinotta F, Alp M, Sulzman F, Wang M. Space radiation risks to the central nervous system. Life Sci Space Res. 2014;2:54–69. doi: 10.1016/j.lssr.2014.06.003. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

