Current Progress and Challenges in the Study of Adjuvants for Oral Vaccines
- PMID: 36607488
- PMCID: PMC9821375
- DOI: 10.1007/s40259-022-00575-1
Current Progress and Challenges in the Study of Adjuvants for Oral Vaccines
Abstract
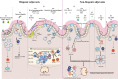
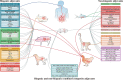
Over the past 20 years, a variety of potential adjuvants have been studied to enhance the effect of oral vaccines in the intestinal mucosal immune system; however, no licensed adjuvant for clinical application in oral vaccines is available. In this review, we systematically updated the research progress of oral vaccine adjuvants over the past 2 decades, including biogenic adjuvants, non-biogenic adjuvants, and their multi-type composite adjuvant materials, and introduced their immune mechanisms of adjuvanticity, aiming at providing theoretical basis for developing feasible and effective adjuvants for oral vaccines. Based on these insights, we briefly discussed the challenges in the development of oral vaccine adjuvants and prospects for their future development.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Bingming Ou, Ying Yang, Haihui Lv, Xin Lin, and Minyu Zhang declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- Grant No. 32102703/National Natural Science Foundation of China
- 2019A1515111186/Basic and Applied Basic Research Joint Fund of Guangdong Province
- 202110580008/National College Students' Innovation and Entrepreneurship Training Plan Program in 2021
- S202010580051/College Students' Innovation and Entrepreneurship Training Plan Program of Guangdong Province in 2020
- Functional laboratory of Disease Prevention/Fund of construction of Ecological Poultry Industry Technology System in Guizhou Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

