COVID-19 vaccines adverse events: potential molecular mechanisms
- PMID: 36607502
- PMCID: PMC9821369
- DOI: 10.1007/s12026-023-09357-5
COVID-19 vaccines adverse events: potential molecular mechanisms
Abstract
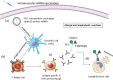
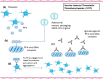
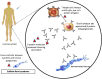
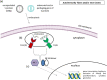
COVID-19 is an infectious disease caused by a single-stranded RNA (ssRNA) virus, known as SARS-CoV-2. The disease, since its first outbreak in Wuhan, China, in December 2019, has led to a global pandemic. The pharmaceutical industry has developed several vaccines, of different vector technologies, against the virus. Of note, among these vaccines, seven have been fully approved by WHO. However, despite the benefits of COVID-19 vaccination, some rare adverse effects have been reported and have been associated with the use of the vaccines developed against SARS-CoV-2, especially those based on mRNA and non-replicating viral vector technology. Rare adverse events reported include allergic and anaphylactic reactions, thrombosis and thrombocytopenia, myocarditis, Bell's palsy, transient myelitis, Guillen-Barre syndrome, recurrences of herpes-zoster, autoimmunity flares, epilepsy, and tachycardia. In this review, we discuss the potential molecular mechanisms leading to these rare adverse events of interest and we also attempt an association with the various vaccine components and platforms. A better understanding of the underlying mechanisms, according to which the vaccines cause side effects, in conjunction with the identification of the vaccine components and/or platforms that are responsible for these reactions, in terms of pharmacovigilance, could probably enable the improvement of future vaccines against COVID-19 and/or even other pathological conditions.
Keywords: Adverse drug reactions; Adverse events; COVID-19 vaccines; Mechanisms; Pharmacovigilance; Side effects.
© 2023. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- COVID19 vaccine tracker [Internet]. Trackvaccines.org. [cited 2022 Sep 15]. Available from: https://covid19.trackvaccines.org/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

