Predicting tolerability of high-dose fentanyl buccal tablets in cancer patients
- PMID: 36608031
- PMCID: PMC9821425
- DOI: 10.1371/journal.pone.0280212
Predicting tolerability of high-dose fentanyl buccal tablets in cancer patients
Abstract
Background & aims: Fentanyl buccal tablets (FBTs) are a rapid-onset opioid indicated for breakthrough cancer pain (BTcP) and FBT titration is needed to optimize BTcP management. We aimed to predict which patients could tolerate a high dose of FBT (400 μg or more at a time).
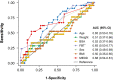
Methods: A retrospective analysis was performed to assess the final FBT dose. The final FBT doses were compared according to the clinical features. The prediction accuracy of patients tolerant of 400 μg or higher FBT was compared using the area under the receiver operating characteristic (ROC) curves. A risk scoring model based on the odds ratio (OR) was developed from the final multivariable model, and patients were assigned into two groups: low tolerance (0-1 point) and high tolerance (2-3 points).
Results: Among 131 patients, the most frequently effective dose of FBT was 200 μg (54%), followed by 100 μg (30%). The median value of morphine equivalent daily doses (MEDD) was 60 mg/day, and the most common daily use was 3-4 times/day. In multivariable analysis, male sex, younger age, and use of FBTs three or more times per day were independently associated with high-dose FBT. According to the risk scoring model, the patients with a final FBT of 400 μg or higher were significantly more in the high tolerance group (17%) compared to the low tolerance group (3%; p = 0.023).
Conclusions: According to the dose relationship between the final FBT dose and the clinical features, three factors (sex, age, daily use of FBT) were independently associated with the final dose of FBT. Our risk score model could help predict tolerance to high-dose FBT and guide the titration plan for BTcP.
Copyright: © 2023 Kwon et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Conflicts of interest relevant to this article was not reported.
Figures
References
-
- Coluzzi PH, Schwartzberg L, Conroy JD, Charapata S, Gay M, Busch MA, et al.. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR). Pain. 2001;91(1–2):123–30. Epub 2001/03/10. doi: 10.1016/s0304-3959(00)00427-9 . - DOI - PubMed
-
- Mercadante S, Radbruch L, Davies A, Poulain P, Sitte T, Perkins P, et al.. A comparison of intranasal fentanyl spray with oral transmucosal fentanyl citrate for the treatment of breakthrough cancer pain: an open-label, randomised, crossover trial. Current medical research and opinion. 2009;25(11):2805–15. Epub 2009/10/02. doi: 10.1185/03007990903336135 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

