Novel modelling approaches to predict the role of antivirals in reducing influenza transmission
- PMID: 36608108
- PMCID: PMC9876374
- DOI: 10.1371/journal.pcbi.1010797
Novel modelling approaches to predict the role of antivirals in reducing influenza transmission
Abstract
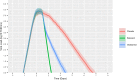
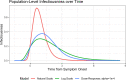
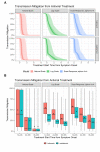
To aid understanding of the effect of antiviral treatment on population-level influenza transmission, we used a novel pharmacokinetic-viral kinetic transmission model to test the correlation between nasal viral load and infectiousness, and to evaluate the impact that timing of treatment with the antivirals oseltamivir or baloxavir has on influenza transmission. The model was run under three candidate profiles whereby infectiousness was assumed to be proportional to viral titer on a natural-scale, log-scale, or dose-response model. Viral kinetic profiles in the presence and absence of antiviral treatment were compared for each individual (N = 1000 simulated individuals); subsequently, viral transmission mitigation was calculated. The predicted transmission mitigation was greater with earlier administration of antiviral treatment, and with baloxavir versus oseltamivir. When treatment was initiated 12-24 hours post symptom onset, the predicted transmission mitigation was 39.9-56.4% for baloxavir and 26.6-38.3% for oseltamivir depending on the infectiousness profile. When treatment was initiated 36-48 hours post symptom onset, the predicted transmission mitigation decreased to 0.8-28.3% for baloxavir and 0.8-19.9% for oseltamivir. Model estimates were compared with clinical data from the BLOCKSTONE post-exposure prophylaxis study, which indicated the log-scale model for infectiousness best fit the observed data and that baloxavir affords greater reductions in secondary case rates compared with neuraminidase inhibitors. These findings suggest a role for baloxavir and oseltamivir in reducing influenza transmission when treatment is initiated within 48 hours of symptom onset in the index patient.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors of this manuscript have the following competing interests: JA, MC, LMTR, DPD, CJA, and DY W are employees of the Office of the Assistant Secretary for Preparedness and Response. ALD, KK, BC, SJ, and JEC are employees and shareholders of F. Hoffmann-La Roche Ltd. LMTR and CJA are employees of Leidos, Inc. ALD, KK, BC, SJ, and JEC are employees and shareholders of F. Hoffmann-La Roche Ltd.
Figures


References
-
- Troeger CE, Blacker BF, Khalil IA, Zimsen SR, Albertson SB, Abate D, et al.. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. The Lancet Respiratory Medicine. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X - DOI - PMC - PubMed
-
- United States Centers for Disease Control and Prevention. Disease Burden of Flu 2022 [cited 2022 12 October]. Available from: https://www.cdc.gov/flu/about/burden/index.html.
-
- Ison MG, Portsmouth S, Yoshida Y, Shishido T, Mitchener M, Tsuchiya K, et al.. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204–14. Epub 2020/06/12. doi: 10.1016/S1473-3099(20)30004-9 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

