Neurexin-2: An inhibitory neurexin that restricts excitatory synapse formation in the hippocampus
- PMID: 36608123
- PMCID: PMC9821874
- DOI: 10.1126/sciadv.add8856
Neurexin-2: An inhibitory neurexin that restricts excitatory synapse formation in the hippocampus
Expression of concern in
-
Editorial expression of concern.Sci Adv. 2025 Oct 3;11(40):eaec3110. doi: 10.1126/sciadv.aec3110. Epub 2025 Oct 1. Sci Adv. 2025. PMID: 41032620 Free PMC article. No abstract available.
Abstract
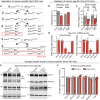
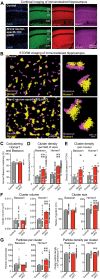
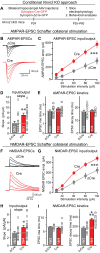
Neurexins are widely thought to promote synapse formation and to organize synapse properties. Here we found that in contrast to neurexin-1 and neurexin-3, neurexin-2 unexpectedly restricts synapse formation. In the hippocampus, constitutive or neuron-specific deletions of neurexin-2 nearly doubled the strength of excitatory CA3➔CA1 region synaptic connections and markedly increased their release probability. No effect on inhibitory synapses was detected. Stochastic optical reconstruction microscopy (STORM) superresolution microscopy revealed that the neuron-specific neurexin-2 deletion elevated the density of excitatory CA1 region synapses nearly twofold. Moreover, hippocampal neurexin-2 deletions also increased synaptic connectivity in the CA1 region when induced in mature mice and impaired the cognitive flexibility of spatial memory. Thus, neurexin-2 controls the dynamics of hippocampal synaptic circuits by repressing synapse assembly throughout life, a restrictive function that markedly differs from that of neurexin-1 and neurexin-3 and of other synaptic adhesion molecules, suggesting that neurexins evolutionarily diverged into opposing pro- and antisynaptogenic organizers.
Figures



References
-
- J. R. Sanes, S. L. Zipursky, Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 536–556 (2020). - PubMed
-
- H. Y. Kim, J. W. Um, J. Ko, Proper synaptic adhesion signaling in the control of neural circuit architecture and brain function. Prog. Neurobiol. 200, 101983 (2021). - PubMed
-
- S. Jang, H. Lee, E. Kim, Synaptic adhesion molecules and excitatory synaptic transmission. Curr. Opin. Neurobiol. 45, 45–50 (2017). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

