Genetically Encoded RNA-Based Bioluminescence Resonance Energy Transfer (BRET) Sensors
- PMID: 36608281
- PMCID: PMC10630924
- DOI: 10.1021/acssensors.2c02213
Genetically Encoded RNA-Based Bioluminescence Resonance Energy Transfer (BRET) Sensors
Abstract
RNA-based nanostructures and molecular devices have become popular for developing biosensors and genetic regulators. These programmable RNA nanodevices can be genetically encoded and modularly engineered to detect various cellular targets and then induce output signals, most often a fluorescence readout. Although powerful, the high reliance of fluorescence on the external excitation light raises concerns about its high background, photobleaching, and phototoxicity. Bioluminescence signals can be an ideal complementary readout for these genetically encoded RNA nanodevices. However, RNA-based real-time bioluminescent reporters have been rarely developed. In this study, we reported the first type of genetically encoded RNA-based bioluminescence resonance energy transfer (BRET) sensors that can be used for real-time target detection in living cells. By coupling a luciferase bioluminescence donor with a fluorogenic RNA-based acceptor, our BRET system can be modularly designed to image and detect various cellular analytes. We expect that this novel RNA-based bioluminescent system can be potentially used broadly in bioanalysis and nanomedicine for engineering biosensors, characterizing cellular RNA-protein interactions, and high-throughput screening or in vivo imaging.
Keywords: bioluminescence resonance energy transfer; bioluminescent sensors; fluorogenic RNA aptamers.
Figures



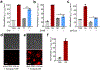
References
-
- Syed AJ; Anderson JC Applications of bioluminescence in biotechnology and beyond. Chem. Soc. Rev 2021, 50 (9), 5668–5705. - PubMed
-
- Wilson T; Hastings JW Bioluminescence. Curr. Rev. Cell Dev. Biol 1998, 14, 197–230. - PubMed
-
- Ozawa T; Yoshimura H; Kim SB Advances in fluorescence and bioluminescence imaging. Anal. Chem 2013, 85 (2), 590–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

