Blue light irradiation exerts anti-viral and anti-inflammatory properties against herpes simplex virus type 1 infection
- PMID: 36608399
- PMCID: PMC9771843
- DOI: 10.1016/j.jphotobiol.2022.112632
Blue light irradiation exerts anti-viral and anti-inflammatory properties against herpes simplex virus type 1 infection
Erratum in
-
Corrigendum to "Blue light irradiation exerts anti-viral and anti-inflammatory properties against herpes simplex virus type 1 infection" [Journal of Photochemistry and Photobiology B: Biology Volume 239 (2023) 112632].J Photochem Photobiol B. 2023 May;242:112684. doi: 10.1016/j.jphotobiol.2023.112684. Epub 2023 Mar 10. J Photochem Photobiol B. 2023. PMID: 36906983 Free PMC article. No abstract available.
Abstract
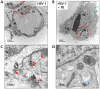
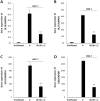
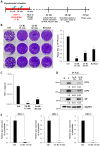
The aim of this study was to investigate the antiviral and anti-inflammatory functions of blue light (BL) in cutaneous viral infections. Previously, we examined the photo-biogoverning role of 450 nm BL in SARS-CoV-2-infected cells, which showed that photo-energy could inhibit viral activation depending on the number of photons. However, the communication network between photo-energy irradiation and immune cells involved in viral infections has not been clarified. We verified viral activation, inflammatory responses, and relevant downstream cascades caused by human simplex virus type I (HSV-1) after BL irradiation. To examine the antiviral effect of BL, we further tested whether BL could disturb viral absorption or entry into host cells. The results showed that BL irradiation, but not green light (GL) exposure, specifically decreased plaque-forming activity and viral copy numbers in HSV-1-infected cells. Accumulated BL irradiation inhibited the localization of viral proteins and the RNA expression of characteristic viral genes such as UL19, UL27, and US6, thus exerting to an anti-viral effect. The results also showed that BL exposure during viral absorption interfered with viral entry or destroyed the virus, as assessed by plaque formation and quantitative PCR assays. The levels of the pro-inflammatory mediators interleukin (IL)-18 and IL-1β in M1-polarized macrophages were increased by HSV-1 infection. However, these increases were attenuated by BL irradiation. Importantly, BL irradiation decreased cGAS and STING expression, as well as downstream NF-κB p65, in M1-polarized HSV-1-infected macrophages, demonstrating anti-viral and anti-inflammatory properties. These findings suggest that BL could serve as an anti-viral and anti-inflammatory therapeutic candidate to treat HSV-1 infections.
Keywords: Blue light; Herpes simplex virus-1; Inflammatory factors; Viral replication.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest All authors declare that they have no conflicts of interest.
Figures





References
-
- Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. - PubMed
-
- Steiner I., Benninger F. Update on herpes virus infections of the nervous system. Curr. Neurol. Neurosci. Rep. 2013;13:414. - PubMed
-
- Kukhanova M.K., Korovina A.N., Kochetkov S.N. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc) 2014;79:1635–1652. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

