Steady-state estradiol triggers a unique innate immune response to allergen resulting in increased airway resistance
- PMID: 36609358
- PMCID: PMC9817388
- DOI: 10.1186/s13293-022-00483-7
Steady-state estradiol triggers a unique innate immune response to allergen resulting in increased airway resistance
Abstract
Rationale: Asthma is a chronic airway condition that occurs more often in women than men during reproductive years. Population studies have collectively shown that long-term use of oral contraceptives decreased the onset of asthma in women of reproductive age. In the current study, we hypothesized that steady-state levels of estrogen would reduce airway inflammation and airway hyperresponsiveness to methacholine challenge.
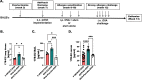
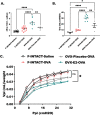
Methods: Ovariectomized BALB/c mice (Ovx) were implanted with subcutaneous hormone pellets (estrogen, OVX-E2) that deliver consistent levels of estrogen [68 ± 2 pg/mL], or placebo pellets (OVX-Placebo), followed by ovalbumin sensitization and challenge. In conjunction with methacholine challenge, immune phenotyping was performed to correlate inflammatory proteins and immune populations with better or worse pulmonary outcomes measured by invasive pulmonary mechanics techniques.
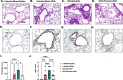
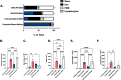
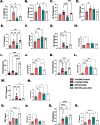
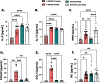
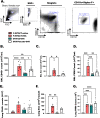
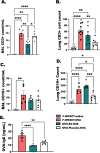
Results: Histologic analysis showed an increase in total cell infiltration and mucus staining around the airways leading to an increased inflammatory score in ovarectomized (OVX) animals with steady-state estrogen pellets (OVX-E2-OVA) as compared to other groups including female-sham operated (F-INTACT-OVA) and OVX implanted with a placebo pellet (OVX-Pl-OVA). Airway resistance (Rrs) and lung elastance (Ers) were increased in OVX-E2-OVA in comparison to F-INTACT-OVA following aerosolized intratracheal methacholine challenges. Immune phenotyping revealed that steady-state estrogen reduced CD3+ T cells, CD19+ B cells, ILC2 and eosinophils in the BAL across all experiments. While these commonly described allergic cells were reduced in the BAL, or airways, we found no changes in neutrophils, CD3+ T cells or CD19+ B cells in the remaining lung tissue. Similarly, inflammatory cytokines (IL-5 and IL-13) were also decreased in OVX-E2-OVA-treated animals in comparison to Female-INTACT-OVA mice in the BAL, but in the lung tissue IL-5, IL-13 and IL-33 were comparable in OVX-E2-OVA and F-INTACT OVA mice. ILC2 were sorted from the lungs and stimulated with exogenous IL-33. These ILC2 had reduced cytokine and chemokine expression when they were isolated from OVX-E2-OVA animals, indicating that steady-state estrogen suppresses IL-33-mediated activation of ILC2.
Conclusions: Therapeutically targeting estrogen receptors may have a limiting effect on eosinophils, ILC2 and potentially other immune populations that may improve asthma symptoms in those females that experience perimenstrual worsening of asthma, with the caveat, that long-term use of estrogens or hormone receptor modulators may be detrimental to the lung microenvironment over time.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures

Similar articles
-
Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses.Am J Physiol Lung Cell Mol Physiol. 2024 Jul 1;327(1):L65-L78. doi: 10.1152/ajplung.00207.2023. Epub 2024 Apr 23. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38651968 Free PMC article.
-
IL-33 induces NF-κB activation in ILC2 that can be suppressed by in vivo and ex vivo 17β-estradiol.Front Allergy. 2022 Nov 25;3:1062412. doi: 10.3389/falgy.2022.1062412. eCollection 2022. Front Allergy. 2022. PMID: 36506643 Free PMC article.
-
Interleukin-22 attenuates allergic airway inflammation in ovalbumin-induced asthma mouse model.BMC Pulm Med. 2021 Nov 26;21(1):385. doi: 10.1186/s12890-021-01698-x. BMC Pulm Med. 2021. PMID: 34836520 Free PMC article.
-
Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice.Biosci Rep. 2019 Jan 8;39(1):BSR20181117. doi: 10.1042/BSR20181117. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30373775 Free PMC article.
-
Sex-Based Differences in Asthma: Pathophysiology, Hormonal Influence, and Genetic Mechanisms.Int J Mol Sci. 2025 May 30;26(11):5288. doi: 10.3390/ijms26115288. Int J Mol Sci. 2025. PMID: 40508095 Free PMC article. Review.
Cited by
-
Sex differences in airway disease: estrogen and airway surface liquid dynamics.Biol Sex Differ. 2024 Jul 18;15(1):56. doi: 10.1186/s13293-024-00633-z. Biol Sex Differ. 2024. PMID: 39026347 Free PMC article. Review.
-
Progesterone amplifies allergic inflammation and airway pathology in association with higher lung ILC2 responses.Am J Physiol Lung Cell Mol Physiol. 2024 Jul 1;327(1):L65-L78. doi: 10.1152/ajplung.00207.2023. Epub 2024 Apr 23. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38651968 Free PMC article.
-
Neuroinflammation is dependent on sex and ovarian hormone presence following acute woodsmoke exposure.Sci Rep. 2024 Jun 6;14(1):12995. doi: 10.1038/s41598-024-63562-2. Sci Rep. 2024. PMID: 38844478 Free PMC article.
References
-
- Padem N, Saltoun C. Classification of asthma. Allergy Asthma Proc. 2019;40(6):385–388. - PubMed
-
- Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. 2018;198(8):1012–1020. - PubMed
-
- Ruppel GL, Enright PL. Pulmonary function testing. Respir Care. 2012;57(1):165–175. - PubMed
-
- Lang JE, Bunnell HT, Lima JJ, Hossain MJ, Wysocki T, Bacharier L, et al. Effects of age, sex, race/ethnicity, and allergy status in obesity-related pediatric asthma. Pediatr Pulmonol. 2019;54(11):1684–1693. - PubMed
-
- Lang JE. Obesity and childhood asthma. Curr Opin Pulm Med. 2019;25(1):34–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

