Structure of the Sec14 domain of Kalirin reveals a distinct class of lipid-binding module in RhoGEFs
- PMID: 36609407
- PMCID: PMC9823006
- DOI: 10.1038/s41467-022-35678-4
Structure of the Sec14 domain of Kalirin reveals a distinct class of lipid-binding module in RhoGEFs
Abstract
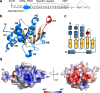
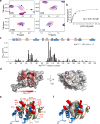
Gated entry of lipophilic ligands into the enclosed hydrophobic pocket in stand-alone Sec14 domain proteins often links lipid metabolism to membrane trafficking. Similar domains occur in multidomain mammalian proteins that activate small GTPases and regulate actin dynamics. The neuronal RhoGEF Kalirin, a central regulator of cytoskeletal dynamics, contains a Sec14 domain (KalbSec14) followed by multiple spectrin-like repeats and catalytic domains. Previous studies demonstrated that Kalirin lacking its Sec14 domain fails to maintain cell morphology or dendritic spine length, yet whether and how KalbSec14 interacts with lipids remain unknown. Here, we report the structural and biochemical characterization of KalbSec14. KalbSec14 adopts a closed conformation, sealing off the canonical ligand entry site, and instead employs a surface groove to bind a limited set of lysophospholipids. The low-affinity interactions of KalbSec14 with lysolipids are expected to serve as a general model for the regulation of Rho signaling by other Sec14-containing Rho activators.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

