Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression
- PMID: 36609449
- PMCID: PMC9817303
- DOI: 10.1186/s13046-022-02586-w
Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression
Erratum in
-
Correction: Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression.J Exp Clin Cancer Res. 2023 Apr 24;42(1):98. doi: 10.1186/s13046-023-02676-3. J Exp Clin Cancer Res. 2023. PMID: 37095515 Free PMC article. No abstract available.
Abstract
Background: N4-acetylcytidine (ac4C), a widespread modification in human mRNAs that is catalyzed by the N-acetyltransferase 10 (NAT10) enzyme, plays an important role in promoting mRNA stability and translation. However, the biological functions and regulatory mechanisms of NAT10-mediated ac4C were poorly defined.
Methods: ac4C mRNA modification status and NAT10 expression levels were analyzed in gastric cancer (GC) samples and compared with the corresponding normal tissues. The biological role of NAT10-mediated ac4C and its upstream and downstream regulatory mechanisms were determined in vitro and in vivo. The therapeutic potential of targeting NAT10 in GC was further explored.
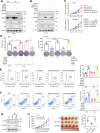
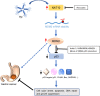
Results: Here, we demonstrated that both ac4C mRNA modification and its acetyltransferase NAT10 were increased in GC, and increased NAT10 expression was associated with disease progression and poor patient prognosis. Functionally, we found that NAT10 promoted cellular G2/M phase progression, proliferation and tumorigenicity of GC in an ac4C-depedent manner. Mechanistic analyses demonstrated that NAT10 mediated ac4C acetylation of MDM2 transcript and subsequently stabilized MDM2 mRNA, leading to its upregulation and p53 downregulation and thereby facilitating gastric carcinogenesis. In addition, Helicobacter pylori (Hp) infection contributed to NAT10 induction, causing MDM2 overexpression and subsequent p53 degradation. Further investigations revealed that targeting NAT10 with Remodelin showed anti-cancer activity in GC and augmented the anti-tumor activity of MDM2 inhibitors in p53 wild-type GC.
Conclusions: These results suggest the critical role of NAT10-mediated ac4C modification in GC oncogenesis and reveal a previously unrecognized signaling cascade involving the Hp-NAT10-MDM2-p53 axis during GC development.
Keywords: Gastric cancer; Helicobacter pylori; MDM2; N-acetyltransferase 10; N4-acetylcytidine; p53.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures






Similar articles
-
Targeting N4-acetylcytidine suppresses hepatocellular carcinoma progression by repressing eEF2-mediated HMGB2 mRNA translation.Cancer Commun (Lond). 2024 Sep;44(9):1018-1041. doi: 10.1002/cac2.12595. Epub 2024 Jul 19. Cancer Commun (Lond). 2024. PMID: 39030964 Free PMC article.
-
NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression.Clin Transl Med. 2022 May;12(5):e738. doi: 10.1002/ctm2.738. Clin Transl Med. 2022. PMID: 35522942 Free PMC article.
-
N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA.Cancer Commun (Lond). 2022 Dec;42(12):1347-1366. doi: 10.1002/cac2.12363. Epub 2022 Oct 8. Cancer Commun (Lond). 2022. PMID: 36209353 Free PMC article.
-
The emerging roles of ac4C acetylation "writer" NAT10 in tumorigenesis: A comprehensive review.Int J Biol Macromol. 2024 Jan;254(Pt 2):127789. doi: 10.1016/j.ijbiomac.2023.127789. Epub 2023 Nov 4. Int J Biol Macromol. 2024. PMID: 37926318 Review.
-
The role and mechanism of NAT10-mediated ac4C modification in tumor development and progression.MedComm (2020). 2024 Dec 4;5(12):e70026. doi: 10.1002/mco2.70026. eCollection 2024 Dec. MedComm (2020). 2024. PMID: 39640362 Free PMC article. Review.
Cited by
-
CircMAST1 inhibits cervical cancer progression by hindering the N4-acetylcytidine modification of YAP mRNA.Cell Mol Biol Lett. 2024 Feb 8;29(1):25. doi: 10.1186/s11658-024-00540-6. Cell Mol Biol Lett. 2024. PMID: 38331765 Free PMC article.
-
Dissecting the oncogenic properties of essential RNA-modifying enzymes: a focus on NAT10.Oncogene. 2024 Apr;43(15):1077-1086. doi: 10.1038/s41388-024-02975-9. Epub 2024 Feb 26. Oncogene. 2024. PMID: 38409550 Free PMC article. Review.
-
Emerging Role of NAT10 as ac4C Writer in Inflammatory Diseases: Mechanisms and Therapeutic Applications.Curr Drug Targets. 2025;26(4):282-294. doi: 10.2174/0113894501346709241202110834. Curr Drug Targets. 2025. PMID: 39633518 Review.
-
Detection of ac4C in human mRNA is preserved upon data reassessment.Mol Cell. 2024 Apr 18;84(8):1611-1625.e3. doi: 10.1016/j.molcel.2024.03.018. Mol Cell. 2024. PMID: 38640896 Free PMC article.
-
Single-cell imaging of N4-acetylcytidine-modified RNA using fluorine metabolic labeling mediated proximity ligation assay.Nucleic Acids Res. 2025 May 22;53(10):gkaf464. doi: 10.1093/nar/gkaf464. Nucleic Acids Res. 2025. PMID: 40464684 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

