Indolethylamine N-methyltransferase (INMT) is not essential for endogenous tryptamine-dependent methylation activity in rats
- PMID: 36609666
- PMCID: PMC9822953
- DOI: 10.1038/s41598-023-27538-y
Indolethylamine N-methyltransferase (INMT) is not essential for endogenous tryptamine-dependent methylation activity in rats
Abstract
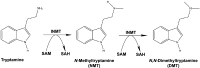
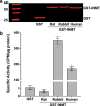
Indolethylamine N-methyltransferase (INMT) is a transmethylation enzyme that utilizes the methyl donor S-adenosyl-L-methionine to transfer methyl groups to amino groups of small molecule acceptor compounds. INMT is best known for its role in the biosynthesis of N,N-Dimethyltryptamine (DMT), a psychedelic compound found in mammalian brain and other tissues. In mammals, biosynthesis of DMT is thought to occur via the double methylation of tryptamine, where INMT first catalyzes the biosynthesis of N-methyltryptamine (NMT) and then DMT. However, it is unknown whether INMT is necessary for the biosynthesis of endogenous DMT. To test this, we generated a novel INMT-knockout rat model and studied tryptamine methylation using radiometric enzyme assays, thin-layer chromatography, and ultra-high-performance liquid chromatography tandem mass spectrometry. We also studied tryptamine methylation in recombinant rat, rabbit, and human INMT. We report that brain and lung tissues from both wild type and INMT-knockout rats show equal levels of tryptamine-dependent activity, but that the enzymatic products are neither NMT nor DMT. In addition, rat INMT was not sufficient for NMT or DMT biosynthesis. These results suggest an alternative enzymatic pathway for DMT biosynthesis in rats. This work motivates the investigation of novel pathways for endogenous DMT biosynthesis in mammals.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Noncompetitive inhibition of indolethylamine-N-methyltransferase by N,N-dimethyltryptamine and N,N-dimethylaminopropyltryptamine.Biochemistry. 2014 May 13;53(18):2956-65. doi: 10.1021/bi500175p. Epub 2014 Apr 28. Biochemistry. 2014. PMID: 24730580 Free PMC article.
-
Biosynthesis and Extracellular Concentrations of N,N-dimethyltryptamine (DMT) in Mammalian Brain.Sci Rep. 2019 Jun 27;9(1):9333. doi: 10.1038/s41598-019-45812-w. Sci Rep. 2019. PMID: 31249368 Free PMC article.
-
Indolethylamine-N-methyltransferase Polymorphisms: Genetic and Biochemical Approaches for Study of Endogenous N,N,-dimethyltryptamine.Front Neurosci. 2018 Apr 23;12:232. doi: 10.3389/fnins.2018.00232. eCollection 2018. Front Neurosci. 2018. PMID: 29740267 Free PMC article. Review.
-
Rabbit lung indolethylamine N-methyltransferase. cDNA and gene cloning and characterization.J Biol Chem. 1998 Dec 18;273(51):34502-10. doi: 10.1074/jbc.273.51.34502. J Biol Chem. 1998. PMID: 9852119
-
Hypothetical biosynthetic pathways of pharmaceutically potential hallucinogenic metabolites in Myristicaceae, mechanistic convergence and co-evolutionary trends in plants and humans.Phytochemistry. 2024 Feb;218:113928. doi: 10.1016/j.phytochem.2023.113928. Epub 2023 Nov 29. Phytochemistry. 2024. PMID: 38035973 Review.
Cited by
-
Single-cell analysis of mesenchymal cells in permeable neural vasculature reveals novel diverse subpopulations of fibroblasts.Fluids Barriers CNS. 2024 Apr 5;21(1):31. doi: 10.1186/s12987-024-00535-7. Fluids Barriers CNS. 2024. PMID: 38575991 Free PMC article.
-
The evolution of N, N-Dimethyltryptamine: from metabolic pathways to brain connectivity.Psychopharmacology (Berl). 2025 Sep;242(9):1931-1953. doi: 10.1007/s00213-025-06777-z. Epub 2025 Apr 11. Psychopharmacology (Berl). 2025. PMID: 40210737 Review.
-
Why N,N-dimethyltryptamine matters: unique features and therapeutic potential beyond classical psychedelics.Front Psychiatry. 2024 Nov 6;15:1485337. doi: 10.3389/fpsyt.2024.1485337. eCollection 2024. Front Psychiatry. 2024. PMID: 39568756 Free PMC article.
-
Neurobiological research on N,N-dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors.Cell Mol Life Sci. 2024 Sep 10;81(1):395. doi: 10.1007/s00018-024-05353-6. Cell Mol Life Sci. 2024. PMID: 39254764 Free PMC article. Review.
References
-
- Axelrod J. The enzymatic N-methylation of serotonin and other amines. J. Pharmacol. Exp. Ther. 1962;138:28–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

