Asymmetric cell division in plant development
- PMID: 36610013
- PMCID: PMC9975081
- DOI: 10.1111/jipb.13446
Asymmetric cell division in plant development
Abstract
Asymmetric cell division (ACD) is a fundamental process that generates new cell types during development in eukaryotic species. In plant development, post-embryonic organogenesis driven by ACD is universal and more important than in animals, in which organ pattern is preset during embryogenesis. Thus, plant development provides a powerful system to study molecular mechanisms underlying ACD. During the past decade, tremendous progress has been made in our understanding of the key components and mechanisms involved in this important process in plants. Here, we present an overview of how ACD is determined and regulated in multiple biological processes in plant development and compare their conservation and specificity among different model cell systems. We also summarize the molecular roles and mechanisms of the phytohormones in the regulation of plant ACD. Finally, we conclude with the overarching paradigms and principles that govern plant ACD and consider how new technologies can be exploited to fill the knowledge gaps and make new advances in the field.
Keywords: asymmetric cell division; peptide signaling; phytohormonal signaling; plant development; polarity proteins; transcription factors.
© 2023 Institute of Botany, Chinese Academy of Sciences.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Figures
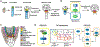



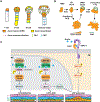

Similar articles
-
Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation.Dev Biol. 2016 Nov 1;419(1):121-131. doi: 10.1016/j.ydbio.2016.07.020. Epub 2016 Jul 28. Dev Biol. 2016. PMID: 27475487 Free PMC article. Review.
-
Where does asymmetry come from? Illustrating principles of polarity and asymmetry establishment in Drosophila neuroblasts.Curr Opin Cell Biol. 2020 Feb;62:70-77. doi: 10.1016/j.ceb.2019.07.018. Epub 2019 Nov 4. Curr Opin Cell Biol. 2020. PMID: 31698250 Review.
-
Polarity and asymmetric cell division in the control of lymphocyte fate decisions and function.Curr Opin Immunol. 2016 Apr;39:143-9. doi: 10.1016/j.coi.2016.02.004. Epub 2016 Mar 2. Curr Opin Immunol. 2016. PMID: 26945468 Review.
-
Principles and mechanisms of asymmetric cell division.Development. 2020 Jun 29;147(13):dev167650. doi: 10.1242/dev.167650. Development. 2020. PMID: 32601056 Free PMC article. Review.
-
Division site determination during asymmetric cell division in plants.Plant Cell. 2022 May 24;34(6):2120-2139. doi: 10.1093/plcell/koac069. Plant Cell. 2022. PMID: 35201345 Free PMC article. Review.
Cited by
-
Multi-scale dynamics influence the division potential of stomatal lineage ground cells in Arabidopsis.Nat Commun. 2025 Mar 17;16(1):2612. doi: 10.1038/s41467-025-57730-9. Nat Commun. 2025. PMID: 40097420 Free PMC article.
-
Twin Embryos in Arabidopsis thaliana KATANIN 1 Mutants.Plants (Basel). 2024 Jul 3;13(13):1824. doi: 10.3390/plants13131824. Plants (Basel). 2024. PMID: 38999664 Free PMC article.
-
A gradient of the HD-Zip regulator Woolly regulates multicellular trichome morphogenesis in tomato.Plant Cell. 2024 May 29;36(6):2375-2392. doi: 10.1093/plcell/koae077. Plant Cell. 2024. PMID: 38470570 Free PMC article.
-
Chemical genetics reveals cross-activation of plant developmental signaling by the immune peptide-receptor pathway.bioRxiv [Preprint]. 2024 Jul 30:2024.07.29.605519. doi: 10.1101/2024.07.29.605519. bioRxiv. 2024. Update in: Sci Adv. 2025 Feb 07;11(6):eads3718. doi: 10.1126/sciadv.ads3718. PMID: 39131359 Free PMC article. Updated. Preprint.
-
Chemical genetics reveals cross-regulation of plant developmental signaling by the immune peptide-receptor pathway.Sci Adv. 2025 Feb 7;11(6):eads3718. doi: 10.1126/sciadv.ads3718. Epub 2025 Feb 5. Sci Adv. 2025. PMID: 39908379 Free PMC article.
References
-
- Abrash EB, and Bergmann DC (2009). Asymmetric cell divisions: a view from plant development. Dev. Cell 16: 783–796. - PubMed
-
- Abrash EB, and Bergmann DC (2010). Regional specification of stomatal production by the putative ligand CHALLAH. Development 137: 447–455. - PubMed
-
- Adrian J, Chang J, Ballenger CE, Bargmann BO, Alassimone J, Davies KA, Lau OS, Matos JL, Hachez C, Lanctot A, Vatén A, Birnbaum KD, and Bergmann DC (2015). Transcriptome dynamics of the stomatal lineage: birth, amplification, and termination of a self-renewing population. Dev. Cell 33: 107–118. - PMC - PubMed
-
- Aichinger E, Kornet N, Friedrich T, and Laux T (2012). Plant stem cell niches. Ann. Rev. Plant Biol 63: 615–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

