Effect of sodium-glucose cotransporter 2 inhibitors on the rate of decline in kidney function: A systematic review and meta-analysis
- PMID: 36610036
- PMCID: PMC9870734
- DOI: 10.1111/1753-0407.13348
Effect of sodium-glucose cotransporter 2 inhibitors on the rate of decline in kidney function: A systematic review and meta-analysis
Abstract
Aim: To investigate the influence of sodium/glucose cotransporter-2 inhibitors (SGLT-2i) on renal function during the course of its administration, particularly in the initial weeks.
Materials and methods: Randomized controlled trials (RCTs) related to SGLT-2i were searched in databases (MEDLINE, EMBASE, and Cochrane Central Register) from the database's inception to August 31, 2021. All RCTs reported the kidney outcomes of SGLT2i versus active or placebo control were included, regardless of the presence of diabetes in the patients and the baseline estimated glomerular filtration rate (eGFR). The Cochrane Collaboration risk of bias tool was used to assess the quality of the included studies. All outcome comparisons were performed using the RevMan 5.4 software.
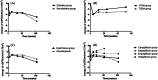
Results: Eleven RCTs with 58 534 participants reporting prespecified renal outcomes were identified. There was no heterogeneity in the baseline eGFR and urine albumin-to-creatinine ratio in the included studies. In the initial 2-4 weeks, there was an acute decline of eGFR in the SGLT-2i group compared with placebo group (weighted mean difference [WMD] -3.35 ml/min/1.73 m2 ; 95% CI, -3.81 to -2.90; I2 = 35%, p = .15); When compared to baseline eGFR in the SGLT-2i group, the WMD was -4.02 ml/min/1.73 m2 (95% confidence interval [CI], -3.61 to -4.44; I2 = 0%, p = .45). The renoprotective effect gradually appeared, and the decline rate of eGFR in the SGLT-2i group was sustained slower than placebo. However, the statistically significant benefit of SGLT-2i did not appear until the 104th week (the second year) (WMD 0.35 ml/min/1.73 m2 , 95% CI, 0.04 to 0.66; I2 = 45%, p = .08). Subgroup analysis showed SGLT-2i had a similar benefit on renal function regardless of baseline eGFR values.
Conclusion: SGLT-2i consistently slowed the deterioration of eGFR since the early stage of administration, even in patients with chronic kidney disease. However, there was an acute decline in eGFR in the initial 2-4 weeks; afterwards the renoprotective effect of SGLT-2i gradually appeared and remained stable in the next few years.
目的: 探讨SGLT-2抑制剂(SGLT-2i)在给药过程中, 特别是最初几周对肾功能的影响。 材料和方法: 从数据库创建至2021年8月31日, 在数据库(MEDLINE、EMBASE和Cochrane Central Register)中搜索与SGLT-2i相关的随机对照试验(RCTs)。所有纳入的RCT研究都报告了SGLT-2i组与安慰剂组的肾脏结局, 无论患者是否存在糖尿病和基线估算肾小球滤过率 (eGFR)。本研究采用Cochrane风险偏倚评估工具来评估纳入研究的质量。所有结果比较均使用RevMan 5.4软件进行。 结果: 共纳入11项RCT研究, 包含的58534名参与者报告了预先指定的肾脏结果。在纳入的研究中, 基线eGFR和UACR没有异质性。在最初的2-4周内, 与安慰剂组相比, SGLT-2i组eGFR快速下降( 加权均数差(WMD) -3.35ml/min/1.73m2 ; 95%CI,-3.81~-2.90; I2 =35%, P=0.15);与SGLT-2i组的基线eGFR相比, WMD为-4.02 ml/min/1.73m2 (95%CI, -3.61~-4.44; I2 =0%, P=0.45)。肾保护作用逐渐显现, SGLT-2i组eGFR下降速度持续慢于安慰剂组。然而, SGLT-2i直到第104周(第二年)才出现统计学上显著的益处(WMD 0.35ml/min/1.73m2 ,95%CI, 0.04~0.66; I2 =45%, P=0.08)。亚组分析显示, 无论eGFR基线值如何, SGLT-2i对肾功能具有相似的益处。 结论: SGLT-2i早期就能持续减缓eGFR的恶化, 即使在慢性肾病患者中也是如此。然而, 在最初的2-4周内, eGFR快速下降, 之后SGLT-2i的肾脏保护作用逐渐显现, 并在接下来的几年保持稳定。.
Keywords: SGLT-2 inhibitors; SGLT-2抑制剂; kidney function; meta-analysis; meta分析; systematic review; 系统综述; 肾功能.
© 2023 The Authors. Journal of Diabetes published by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin. 2009;25(3):671‐681. - PubMed
-
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium‐glucose co‐transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33‐59. - PubMed
-
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752‐772. - PubMed
-
- Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860‐1870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

