Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer
- PMID: 36610262
- PMCID: PMC10024138
- DOI: 10.1016/j.esmoop.2022.100762
Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer
Erratum in
-
Corrigendum to "Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer": [ESMO Open 8 (2023) 100762].ESMO Open. 2024 Jan;9(1):102232. doi: 10.1016/j.esmoop.2023.102232. Epub 2024 Jan 8. ESMO Open. 2024. PMID: 38194883 Free PMC article. No abstract available.
Abstract
Background: We conducted comprehensive clinical and molecular characterization of claudin 18.2 expression (CLDN18.2) in advanced gastric or gastroesophageal junction cancer (GC/GEJC).
Patients and methods: Patients with advanced GC/GEJC who received systemic chemotherapy from October 2015 to December 2019 with available tumor specimens were analyzed. We evaluated clinicopathological features of CLDN18.2 expression with four molecular subtypes: mismatch repair deficient, Epstein-Barr virus-positive, human epidermal growth factor receptor 2-positive, and others. In addition, programmed death-ligand 1 (PD-L1) combined positive score (CPS), genomic alterations, and the expression of immune cell markers were assessed. Clinical outcomes of standard first- or second-line chemotherapy and subsequent anti-programmed cell death protein 1 (anti-PD-1) therapy were also investigated according to CLDN18.2 expression.
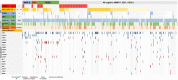
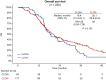
Results: Among 408 patients, CLDN18.2-positive (moderate-to-strong expression in ≥75%) was identified in 98 patients (24.0%) with almost equal distribution in the four molecular subtypes or CPS subgroups. CLDN18.2-positive was associated with Borrmann type 4, KRAS amplification, low CD16, and high CD68 expression. Overall survival with first-line chemotherapy was not significantly different between CLDN18.2-positive and -negative groups [median 18.4 versus 20.1 months; hazard ratio 1.26 (95% confidence interval 0.89-1.78); P = 0.191] regardless of stratification by PD-L1 CPS ≥5. Progression-free survival and objective response rates of first- and second-line chemotherapy, and anti-PD-1 therapy also showed no significant differences according to CLDN18.2 status.
Conclusions: CLDN18.2 expression in advanced GC/GEJC was associated with some clinical and molecular features but had no impact on treatment outcomes with chemotherapy or checkpoint inhibition. CLDN18.2-positive also had no impact on overall survival. This information could be useful to interpret the results from currently ongoing clinical trials of CLDN18.2-targeted therapies for advanced GC/GEJC and to consider a treatment strategy for CLDN18.2-positive GC/GEJC.
Keywords: Epstein–Barr virus; claudin 18.2; gastric cancer; mismatch repair deficiency; zolbetuximab.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


Comment in
-
Claudin18.who? Examining biomarker overlap and outcomes in claudin18.2-positive gastroesophageal adenocarcinomas.ESMO Open. 2023 Apr;8(2):100778. doi: 10.1016/j.esmoop.2022.100778. Epub 2023 Feb 13. ESMO Open. 2023. PMID: 36791669 Free PMC article. No abstract available.
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Ajani J.A., D’Amico T.A., Bentrem D.J., et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:167–192. - PubMed
-
- Cunningham D., Starling N., Rao S., et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
-
- Koizumi W., Narahara H., Hara T., et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. - PubMed
-
- Bang Y.J., van Cutsem E., Feyereislova A., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

