AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection
- PMID: 36610390
- PMCID: PMC9750884
- DOI: 10.1016/j.xcrm.2022.100882
AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection
Abstract
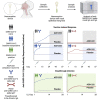
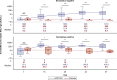
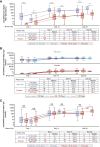
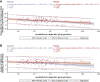
The nasal mucosa is an important initial site of host defense against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, intramuscularly administered vaccines typically do not achieve high antibody titers in the nasal mucosa. We measure anti-SARS-CoV-2 spike immunoglobulin G (IgG) and IgA in nasal epithelial lining fluid (NELF) following intramuscular vaccination of 3,058 participants from the immunogenicity substudy of a phase 3, double-blind, placebo-controlled study of AZD1222 vaccination (ClinicalTrials.gov: NCT04516746). IgG is detected in NELF collected 14 days following the first AZD1222 vaccination. IgG levels increase with a second vaccination and exceed pre-existing levels in baseline-SARS-CoV-2-seropositive participants. Nasal IgG responses are durable and display strong correlations with serum IgG, suggesting serum-to-NELF transudation. AZD1222 induces short-lived increases to pre-existing nasal IgA levels in baseline-seropositive vaccinees. Vaccinees display a robust recall IgG response upon breakthrough infection, with overall magnitudes unaffected by time between vaccination and illness. Mucosal responses correlate with reduced viral loads and shorter durations of viral shedding in saliva.
Keywords: AZD1222; COVID-19 vaccine; ChAdOx1 nCoV-19; SARS-CoV-2 spike antibodies; breakthrough infection; immunoassay; mucosal immune response; nasal antibody; nasal mucosal immunity; serology.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.A.A., D.W., A.M.S., J.M., and E.J.K. are current employees of AstraZeneca and hold or may hold AstraZeneca stock. H.B. and S. Sanikommui are contractors to AstraZeneca via Bogier Consulting. S. Sproule is a contractor to AstraZeneca via Joule/System One. M.E.S. has received research grants from the Bill and Melinda Gates Foundation, Gilead Sciences, Janssen Global Services, LLC, Merck, and Sanofi Pasteur. Inc. A.R.F. has received institutional grants for research from Pfizer, Merck, Sharpe and Dohme, Janssen, and BioFire Diagnostics and has received fees for serving on the Novavax COVID-19 vaccine Data and Safety Monitoring Board.
Figures



References
-
- Lambrecht B.N., Neyt K., van Helden M.J. 4th Edition. Academic Press; 2015. Chapter 94 - The mucosal immune response to respiratory viruses.
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

