Splicing factor-mediated regulation patterns reveals biological characteristics and aid in predicting prognosis in acute myeloid leukemia
- PMID: 36611187
- PMCID: PMC9824960
- DOI: 10.1186/s12967-022-03868-9
Splicing factor-mediated regulation patterns reveals biological characteristics and aid in predicting prognosis in acute myeloid leukemia
Abstract
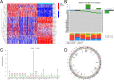
Background: Alternative splicing (AS) of RNA is a fundamental biological process that shapes protein diversity. Many non-characteristic AS events are involved in the onset and development of acute myeloid leukemia (AML). Abnormal alterations in splicing factors (SFs), which regulate the onset of AS events, affect the process of splicing regulation. Hence, it is important to explore the relationship between SFs and the clinical features and biological processes of patients with AML.
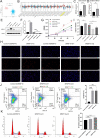
Methods: This study focused on SFs of the classical heterogeneous nuclear ribonucleoprotein (hnRNP) family and arginine and serine/arginine-rich (SR) splicing factor family. We explored the relationship between the regulation patterns associated with the expression of SFs and clinicopathological factors and biological behaviors of AML based on a multi-omics approach. The biological functions of SRSF10 in AML were further analyzed using clinical samples and in vitro experiments.
Results: Most SFs were upregulated in AML samples and were associated with poor prognosis. The four splicing regulation patterns were characterized by differences in immune function, tumor mutation, signaling pathway activity, prognosis, and predicted response to chemotherapy and immunotherapy. A risk score model was constructed and validated as an independent prognostic factor for AML. Overall survival was significantly shorter in the high-risk score group. In addition, we confirmed that SRSF10 expression was significantly up-regulated in clinical samples of AML, and knockdown of SRSF10 inhibited the proliferation of AML cells and promoted apoptosis and G1 phase arrest during the cell cycle.
Conclusion: The analysis of splicing regulation patterns can help us better understand the differences in the tumor microenvironment of patients with AML and guide clinical decision-making and prognosis prediction. SRSF10 can be a potential therapeutic target and biomarker for AML.
Keywords: Alternative splicing; Prognosis; SRSF10; Splicing factor; Tumor microenvironment.
© 2023. The Author(s).
Conflict of interest statement
The authors state no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

