Immunophenotype of Measurable Residual Blast Cells as an Additional Prognostic Factor in Adults with B-Cell Acute Lymphoblastic Leukemia
- PMID: 36611312
- PMCID: PMC9818326
- DOI: 10.3390/diagnostics13010021
Immunophenotype of Measurable Residual Blast Cells as an Additional Prognostic Factor in Adults with B-Cell Acute Lymphoblastic Leukemia
Abstract
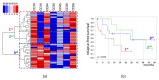
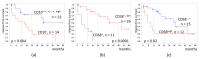
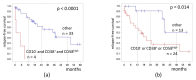
Measurable residual disease (MRD) is a well-known independent prognostic factor in acute leukemias, and multicolor flow cytometry (MFC) is widely used to detect MRD. MFC is able not only to enumerate MRD accurately but also to describe an antigen expression profile of residual blast cells. However, the relationship between MRD immunophenotype and patient survival probability has not yet been studied. We determined the prognostic impact of MRD immunophenotype in adults with B-cell acute lymphoblastic leukemia (B-ALL). In a multicenter study RALL-2016 (NCT03462095), 267 patients were enrolled from 2016 to 2022. MRD was assessed at the end of induction (day 70) in 94 patients with B-ALL by six- or 10-color flow cytometry in the bone marrow specimens. The 4 year relapse-free survival (RFS) was lower in MRD-positive B-ALL patients [37% vs. 78% (p < 0.0001)]. The absence of CD10, positive expression of CD38, and high expression of CD58 on MRD cells worsened the 4 year RFS [19% vs. 51% (p = 0.004), 0% vs. 51% (p < 0.0001), and 21% vs. 40% (p = 0.02), respectively]. The MRD immunophenotype is associated with RFS and could be an additional prognostic factor for B-ALL patients.
Keywords: acute lymphoblastic leukemia; flow cytometry; immunophenotype; measurable residual disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Post-induction Measurable Residual Disease Using Multicolor Flow Cytometry Is Strongly Predictive of Inferior Clinical Outcome in the Real-Life Management of Childhood T-Cell Acute Lymphoblastic Leukemia: A Study of 256 Patients.Front Oncol. 2020 Apr 24;10:577. doi: 10.3389/fonc.2020.00577. eCollection 2020. Front Oncol. 2020. PMID: 32391267 Free PMC article.
-
Early (Day 15 Post Diagnosis) Peripheral Blood Assessment of Measurable Residual Disease in Flow Cytometry is a Strong Predictor of Outcome in Childhood B-Lineage Lymphoblastic Leukemia.Cytometry B Clin Cytom. 2019 Mar;96(2):128-133. doi: 10.1002/cyto.b.21769. Epub 2019 Feb 7. Cytometry B Clin Cytom. 2019. PMID: 30734503
-
Eleven-marker 10-color flow cytometric assessment of measurable residual disease for T-cell acute lymphoblastic leukemia using an approach of exclusion.Cytometry B Clin Cytom. 2021 Jul;100(4):421-433. doi: 10.1002/cyto.b.21939. Epub 2020 Aug 19. Cytometry B Clin Cytom. 2021. PMID: 32812702
-
Minimal/Measurable Residual Disease Detection in Acute Leukemias by Multiparameter Flow Cytometry.Curr Hematol Malig Rep. 2018 Dec;13(6):455-466. doi: 10.1007/s11899-018-0479-1. Curr Hematol Malig Rep. 2018. PMID: 30446941 Review.
-
Measurable Residual Disease in Acute Myeloid Leukemia Using Flow Cytometry: A Review of Where We Are and Where We Are Going.J Clin Med. 2020 Jun 3;9(6):1714. doi: 10.3390/jcm9061714. J Clin Med. 2020. PMID: 32503122 Free PMC article. Review.
Cited by
-
A potential prognostic marker for hematologic neoplasms: CD58.Front Oncol. 2025 Apr 29;15:1586842. doi: 10.3389/fonc.2025.1586842. eCollection 2025. Front Oncol. 2025. PMID: 40365344 Free PMC article. Review.
-
PSG and Other Candidate Genes as Potential Biomarkers of Therapy Resistance in B-ALL: Insights from Chromosomal Microarray Analysis and Machine Learning.Int J Mol Sci. 2025 Aug 1;26(15):7437. doi: 10.3390/ijms26157437. Int J Mol Sci. 2025. PMID: 40806565 Free PMC article.
-
The expression of CD109 in B-cell acute lymphoblastic leukemia and its potential as a promising marker for minimal residual disease (MRD) detection.Clin Exp Med. 2025 Aug 14;25(1):292. doi: 10.1007/s10238-025-01823-8. Clin Exp Med. 2025. PMID: 40810756 Free PMC article.
References
-
- Kuiper R.P., Hoogeveen P.G., Bladergroen R., van Dijk F., Sonneveld E., van Leeuwen F.N., Boer J., Sergeeva I., Feitsma H., den Boer M.L., et al. Minimal residual disease (MRD) detection in acute lymphoblastic leukaemia based on fusion genes and genomic deletions: Towards MRD for all. Br. J. Haematol. 2021;194:888–892. doi: 10.1111/bjh.17744. - DOI - PMC - PubMed
-
- Berry D.A., Zhou S., Higley H., Mukundan L., Fu S., Reaman G.H., Wood B.L., Kelloff G.J., Jessup J.M., Radich J.P. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol. 2017;3:e170580. doi: 10.1001/jamaoncol.2017.0580. - DOI - PMC - PubMed
-
- Flohr T., Schrauder A., Cazzaniga G., Panzer-Grümayer R., van der Velden V., Fischer S., Stanulla M., Basso G., Niggli F.K., Schäfer B.W., et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–782. doi: 10.1038/leu.2008.5. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

